
��ͼA��ʵ������ʯ��ʯ��ϡ������ȡCO2�ij���װ�á���ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ����ͼ�е�Dװ���ռ�һƿ���������CO2���塣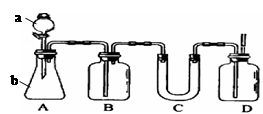
��1��a��b���������Ʒֱ��� �� ��
��2������D�������Ƿ��ռ����˵IJ����ǣ�_________________________________��
��3��B��װ��һ�����ı���NaHCO3Ŀ�ij�ȥA�ӷ���HCl��Ӧ�ķ���ʽ
��4��C��װ��������ȥˮ��������ѡ��������
aŨ���� b�ռ� c��ˮ�Ȼ��� d��ʯ��
��1�� ��Һ©�� �� ��ƿ ��2��ȼ��ľ����������ƿ
��3�� NaHCO3+HCl=NaCl+H2O+CO2 ��4�� C
�������������CO2���岻��ȼ�գ�����ȼ�ŵ�ľ����������ƿ����ľ��Ϩ��������������塣����NaHCO3��CO2����Ӧ���������ڳ��ӡ�Cװ��ΪU�ܣ�����װ����������CO2���������壬�ʲ������ռ�ͼ�ʯ�Ҹ��
���㣺��ѧʵ��
�������Ի�ѧʵ��Ŀ���������ĸ߿��ص㣬�����ڱ�����Ӧע��Ի�ѧ������ʹ�á���ѧʵ������ʵ�鰲ȫ���������ʵ��Ʊ����ռ��ȵ����֪ʶ�Ļ��ۡ�֪ʶ��϶࣬�ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʵ�������һ�ֳ���װ�ã��������ɽ�������ʵ�飮
��ͼ��ʵ�������һ�ֳ���װ�ã��������ɽ�������ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ������2010�������ѧ��9��ģ���⻯ѧ���� ���ͣ�013
|
��ͼ����ȡ�����һ��װ�ã������ôˡ��濪���ã������ͣ��װ����ȡ�������
| |
| [����] | |
A�� |
ʵ�����ô���ʯ��ϡ������ȡCO2 |
B�� |
��ʯ����Ũ��ˮ��NH3 |
C�� |
п����ϡ���������� |
D�� |
��Ƭ���ռ���Һ��H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013ѧ�꺣��ʡ�����и߶���ѧ�ڽ�ѧ������⣨�����Ŀƻ�ѧ���������棩 ���ͣ�ʵ����
��ͼA��ʵ������ʯ��ʯ��ϡ������ȡCO2�ij���װ�á���ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ����ͼ�е�Dװ���ռ�һƿ���������CO2���塣
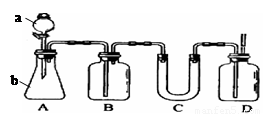
��1��a��b���������Ʒֱ��� �� ��
��2������D�������Ƿ��ռ����˵IJ����ǣ�_________________________________��
��3��B��װ��һ�����ı���NaHCO3Ŀ�ij�ȥA�ӷ���HCl��Ӧ�ķ���ʽ
��4��C��װ��������ȥˮ��������ѡ��������
aŨ���� b�ռ� c��ˮ�Ȼ��� d��ʯ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��ĩ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com