
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100�������֮�ȣ���ʹ�á���ش��������⣺

��1���á�84����Һ�������ʵ���Ũ��ԼΪ________mol��L��1��������С�����һλ��
��2��ijͬѧȡ100 mL�á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ��c��Na������________mol��L��1��
��3����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ������˵����ȷ����________������ĸ����
A������ƿ������ˮϴ����Ӧ��ɺ����������Һ����
B�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
C����Ҫ����NaClO���������Ϊ143.0 g
��4����84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%���ܶ�Ϊ1.84 g��cm��3����Ũ��������2 L 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________mol��L��1��
������Ũ��������Ϊ________mL��
���𰸡�4.0 0.04 B 4.6 250
��������
��1������![]() ���㣻
���㣻
��2������ϡ���������ʵ����ʵ���������
��3������480 mL��NaClO��������Ϊ25%������Һ��Ҫ500mL����ƿ�����һ�����ʵ���Ũ����Һ���Ƶ�ԭ���������
��4�����������Ƕ�Ԫǿ����������ӵ�Ũ�ȣ�����ϡ��������������ʵ������������ҪŨ����������
��1����![]() �ã�c(NaClO)��1000��1.19��25%/74.5 g��mol��1��4.0 mol��L��1��
�ã�c(NaClO)��1000��1.19��25%/74.5 g��mol��1��4.0 mol��L��1��
��2��ϡ��ǰ����Һ��NaClO�����ʵ������䣬��ϡ��100����c(NaClO)��0.04 mol��L��1��c(Na��)��c(NaClO)��0.04 mol��L��1��
��3��A�����ƹ�������Ҫ����ˮ�����Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�ã�������ƿ���ܺ�ɣ�A����
B��δϴ���ձ��Ͳ��������������Ƶ���Һ�����ʵ����ʵ�����С�����ƫ�ͣ�B��ȷ��
C��Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480 mL���ɣ�������ҪNaClO������Ϊ0.5 L��4.0 mol��L��1��74.5 g��mol��1��149.0 g��C����
��ѡB��
��4���ٸ���H2SO4����ɿ�֪����Һ��c(H��)��2c(H2SO4)��4.6 mol��L��1��
��2 L 2.3 mol��L��1��ϡ���������ʵ����ʵ���Ϊ2 L��2.3 mol��L��1��4.6 mol������Ҫ98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������ΪV mL�����У�VmL��1.84g/mL��98%/98g��mol��1��4.6 mol�����V��250��


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
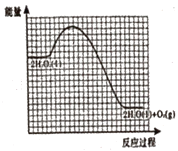
����Ŀ����֪��2H2O2(l)=2H2O(l)+O2(g) ��H=-akJ��mol-1(a��0)
��1��KI��Һ����ΪH2O2�ֽⷴӦ�Ĵ����������̰�����������Ӧ���У�
��.H2O2(l)+I-(aq)=H2O(l)+IO-(aq) ��H1=+bkJ��mol-1(b��0)
��.__ ��H2=__kJ��mol-1���ú�a��b�Ĵ���ʽ��ʾ��
���뽫�������̲�����������ʾ����Ӧ���ǷֽⷴӦ����
����֪����ͬ�����£���Ӧ��ķ�Ӧ����С�ڷ�Ӧ��ķ�Ӧ���ʡ���ͼΪδ�Ӵ���ʱH2O2�ֽⷴӦ������-��Ӧ����ʾ��ͼ������ͼ�л�������KI��Һ��÷�Ӧ������-��Ӧ����ʾ��ͼ___��
��2��H2O2��ˮ��Һ�������ԣ������������£�H2O2H++HO2-��HO2-H++O22-
ij�¶��£������ƽ�ⳣ��K1=1.1��10-11������������Ϊ30%�����ʵ���Ũ��Ϊ10mol��L-1����H2O2ˮ��Һ��pH��__��������H2O2�Ķ������뼰ˮ�ĵ��룩��
��3��Ϊ�о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죨���Ӵ��������ɽ�һ��Ũ�Ⱥ������H2O2�����ܱ������У���ij�¶��£�����һ����ʱ��t���ⶨ����O2�����V��Ȼ��������ʼ�������䣬�ı��¶�![]() ���ظ�����ʵ�顣���V(O2)��ת���ɱ���µ��������T��ϵ���ߡ���������ͼ���ܷ���ʵ��V(O2)��T��ϵ���ߵ���__��ԭ����__��
���ظ�����ʵ�顣���V(O2)��ת���ɱ���µ��������T��ϵ���ߡ���������ͼ���ܷ���ʵ��V(O2)��T��ϵ���ߵ���__��ԭ����__��
A.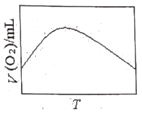 B.
B.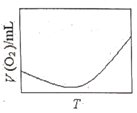
C.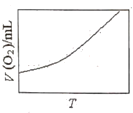 D.
D.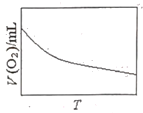
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ȩ(CCl3CHO)������ũҩ��ҽҩ����Ҫ�м��壬ʵ�����Ʊ�������ȩ�ķ�Ӧװ��ʾ��ͼ(����װ��δ����)���й��������£�
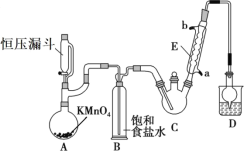
���Ʊ���Ӧԭ����C2H5OH��4Cl2��CCl3CHO��5HCl
��������ʵ���Է��������������������ʣ�
��Է������� | �۵�/�� | �е�/�� | �ܽ��� | |
C2H5OH | 46 | ��114.1 | 78.3 | ��ˮ���� |
CCl3CHO | 147.5 | ��57.5 | 97.8 | ������ˮ���Ҵ� |
CCl3COOH | 163.5 | 58 | 198 | ������ˮ���Ҵ���������ȩ |
C2H5Cl | 64.5 | ��138.7 | 12.3 | ����ˮ���������Ҵ� |
(1)��ѹ©����ʢ�ŵ��Լ���������_____��ʢ��KMnO4������������_____��
(2)��Ӧ������C2H5OH��HCl���ܻ����ɸ�����C2H5Cl��ͬʱCCl3CHO(������ȩ)Ҳ�ܱ������������������CCl3COOH(��������)��д��������ȩ������������������������Ļ�ѧ����ʽ��_____��
(3)����������д���һ��ȱ����_____����������ĺ����_____��װ��B��������______��
(4)��Ӧ��������������Ƚ�C�еĻ������ȴ�����£����÷�Һ�ķ���������������ᡣ����Ϊ�˷����Ƿ����_____(���ǻ��)��ԭ����_____��
(5)�ⶨ��Ʒ���ȣ���ȡ��Ʒ0.36g��ɴ�����Һ������0.1000molL1�����Һ20.00mL���ټ�������Na2CO3��Һ����Ӧ��ȫ�����������Һ��pH��������0.02000molL1Na2S2O3��Һ�ζ����յ㡣��������ƽ��ʵ�飬���ƽ������Na2S2O3��Һ20.00mL�����Ʒ�Ĵ���Ϊ_____(������������λ��Ч����)���ζ�ԭ����CCl3CHO+OH-=CHCl3+HCOO-��HCOO-+I2=H++2I-+CO2��I2+2S2O32-=2I-+S4O62-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ����٤��������ֵ������˵���������()
A. ���³�ѹ�£�4gD2O�к��еĵ�����Ϊ2NA
B. 42gC2H4��C4H8�Ļ�����к�����ԭ����Ϊ6NA
C. 25��ʱ��pH=1��H3PO4��Һ�к���H+��Ϊ0.1NA
D. H2O(g)ͨ��Na2O2(s)ʹ������bgʱ����Ӧ��ת�Ƶĵ�����ΪbNA/2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѪҺ��Ca2����Ũ��һ�����mg/cm3����ʾ����ȡһ�������Ѫ�����������IJ����[(NH4)2C2O4]��Һ�������������(CaC2O4)���������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵò���(H2C2O4)������KMnO4��Һ�ζ����ɲⶨѪҺ��Ʒ��Ca2����Ũ�ȡ�ij�о���ѧϰС���������ʵ�鲽��ⶨѪҺ��Ʒ��Ca2����Ũ�ȡ�
������KMnO4����Һ����ͼ������50 mL KMnO4����Һ�Ĺ���ʾ��ͼ��

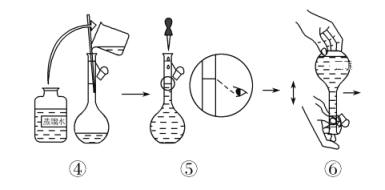
(1)����۲�ͼʾ�жϣ����в���ȷ�IJ�����(�����)________��
(2)����ȷ��50 mL��Һ�����������(������)________��
(3)�����ͼʾ�IJ���������Һ�������Ƶ���ҺŨ�Ƚ�________(�ƫ��ƫС��)��
���ⶨѪҺ��Ʒ��Ca2����Ũ�ȣ���ȡѪ��20.00 mL����������������õ����ᣬ����0.020 mol/L KMnO4��Һ�ζ���ʹ����ת����CO2�ݳ�����ʱ������12.00 mL KMnO4��Һ��
(4)��֪�����KMnO4��Һ��Ӧ�����ӷ���ʽΪ��
2MnO4-��5H2C2O4��6H��===2Mnx����10CO2����8H2O��ʽ�е�x��________��
(5)�������㣬ѪҺ��Ʒ��Ca2����Ũ��Ϊ________mg/cm3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Լ���Ϊԭ�Ϻϳɲ��ֻ�����Ʒ������ͼ(���ַ�Ӧ��������ȥ) ����֪ A �ͼ�������ʯ�ͺ�ú�����ֻ�������ԭ�ϡ�A ����̬��������Һ̬����A �dz�����ֲ���������ڼ���75% ��B ��Һ������ɱ�����¹���������D �Ǹ߷��ӻ����
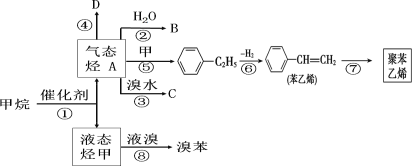
(1)A �Ľṹ��ʽΪ_______��B �й���������Ϊ_____��C ������Ϊ_____��
(2)�ڷ�Ӧ�ڡ��ۡ��ޡ����У����ڼӳɷ�Ӧ����_____������ȡ����Ӧ����_____��(�����)
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��ע����Ӧ������
��Ӧ�ڣ�_____��
��Ӧ�ߣ�_____��
��Ӧ�ࣺ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ڽ���Ԫ�ؼ�~����Ԫ�����ڱ��е����λ�����ұ���ʾ�������ж���ȷ����

A. ԭ�Ӱ뾶������������B. �����ԣ��ף���
C. ����������ԣ�����������D. �������������ף���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B���ֿ���������ɵĻ�������������ʵ�飺
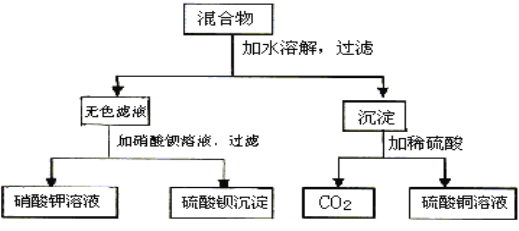
(1)��A��Һ����ɫ�����жϣ�A��B�ֱ���_____________��_____________��(д��ѧʽ)
(2)д��ʵ������з�����Ӧ�����ӷ���ʽ_____________��_____________��_____________��(˳����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̽���糡�������������ӵ�Ǩ�ơ�a��b��c��d ��Ϊʯī�缫���缫���4cm����pH��ֽ�ò�ͬŨ��Na2SO4��Һ�����ʪ����������ʵ�飺

ʵ������
ʱ�� | ��ֽI | ��ֽII |
lmin | a��������ֽ��죬b��������ֽ���� | c��������ֽ��죬d���������� |
10min | ��ɫ������ɫ���������м���չ������ʱ��ɫ��Լ2.7cm����ɫ��Լ1.3cm | ������ɫ��Χ�������ԣ���ֽ����Ϊ��ɫ |
����˵������ȷ����
A. d��������ֽ����
B. a��������ֽ����ԭ���ǣ�2H2O+2e-= H2��+2OH-
C. �Ա���ֽI����ֽII������˵�������Ũ�Ȼ���Ӱ��H+��OH-��Ǩ��
D. ��ֽI������˵�����˻�����H+��Ǩ�����ʱ�OH-��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com