
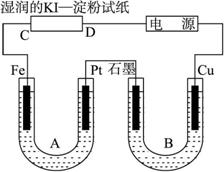
ͼ1-8
(1)A�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________��
(2)��B�й۲쵽��������________________________________________________________��
(3)�����£����ӵ�ʼ��ʱ��ΪTʱ��A��Bװ���й��ռ�������0.168 L(��״��)����������������������Ӧ����������Һ����仯���Բ��ƣ�����Tʱ��A��Һ��pHΪ__________________��
��������ʪ���KI������ֽC�˱�������֪��C��I-������ΪI2����C��Ϊ�����������õ�Դ�Ҷ�Ϊ���������Ϊ�������ɴ˵�Aװ����Fe��Ϊ������PtΪ��������ʵ�ʵ��AgNO3��H2O��A�е���ܷ�ӦΪ4AgNO3+2H2O![]() 4Ag��+O2��+4HNO3��
4Ag��+O2��+4HNO3��
Bװ���� Cu��Ϊ������ʯī��Ϊ������ʵ�ʵ��Cu��H2O���ɴ˵�B������Ϊʯī�缫���������ݲ�����ͭ�缫��Χ��Һ����ɫ��һ��ʱ���U�ι��²�����ɫ����������A��Bװ���ռ���������ֱ���O2��H2����ת�Ƶĵ�������ȣ���n(O2)=![]() n(H2)������n(O2)=
n(H2)������n(O2)=![]() ��
��![]() =0.002 5 mol����A�ܷ�Ӧ��n(HNO3)=4n(O2)=0.010 0 mol
=0.002 5 mol����A�ܷ�Ӧ��n(HNO3)=4n(O2)=0.010 0 mol
�ʣ�H+��=![]() =10-2 mol-1 pH=2
=10-2 mol-1 pH=2
�𰸣�(1)4AgNO3+2H2O![]() 4Ag��+O2��+4HNO3
4Ag��+O2��+4HNO3
(2)ʯī�缫���������ݲ�����ͭ�缫��Χ��Һ����ɫ��һ��ʱ���U�ι��²�����ɫ��������
(3)2



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼװ�ã��ش��������⣺
����ͼװ�ã��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ѡ���ֻ�ѧ�ս̡���ѧ��Ӧԭ�� �ս̰� ���ͣ�022
������ͼ���װ�ã�ͼ��Bװ��ʢ1 L��2 mol��L��1��Na2SO4��Һ��Aװ����ʢ1 L��2 mol��L��1��AgNO3��Һ��ͨ���ʪ���KI������ֽ��C�˱���ɫ�����һ��ʱ����Իش�

(1)A�з�����Ӧ�Ļ�ѧ����ʽΪ_________��
(2)��B�й۲쵽��������_________��
(3)�����£����ӵ�ʼ��ʱ��ΪTʱ��A��Bװ���й��ռ�������0.168 L(��״��)����������������������Ӧ����������Һ����仯���Բ��ƣ�����Tʱ��A��Һ��pHΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)A�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________��
(2)��B�й۲쵽��������________________________________________________________��
(3)�����£����ӵ�ʼ��ʱ��ΪTʱ��A��Bװ���й��ռ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com