




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��B<C<N | B��He<Ne<Ar | C��Si<P<As | D��Na<Mg<Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��32Heԭ�Ӻ��ں���2������ | B��32Heԭ�Ӻ��ں���3������ |
| C��32Heԭ�Ӻ�����3������ | D��32He��42He�����ֲ�ͬ�ĺ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
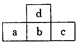
| A��a��b��c������������Ӧˮ���������ǿ����ϵΪc��b��a |
| B��d���⻯���b���⻯���ȶ� |
| C��a��d��ԭ�ӽ�Ͽ��γɴ���������ɻ��һ������ɵ������� |
| D��ԭ�Ӱ뾶��c��b��a��d |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
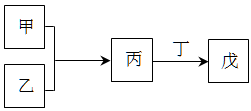
| A�������������ᷴӦ������NaOH��Һ��Ӧ����������������������� |
| B������Ϊ��������ԭ�Ӱ뾶��������Ԫ�صĵ��ʣ�����Ϊ����ֻ��ΪNa2O2 |
| C������������ϲ������̣��ұ�Ϊ18���ӷ��ӣ���Ϊ10���ӷ��ӣ����ҵ�ˮ��Һ���ܾ���Ư������ |
| D�����ס������캬��ͬһ��Ԫ�أ������������и�Ԫ�صĻ��ϼ��ɵ͵��ߵ�˳�����Ϊ���� < �� < �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
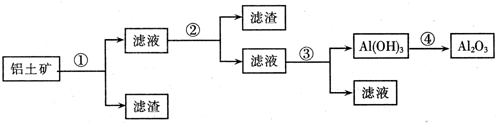
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com