
�����ڹ�ũҵ����������ҪӦ�á�
��1���ٵ������ڹ�ҵ�ϳɰ���д�������ĵ���ʽ ��
��NH3���ȶ��Ա�PH3 ����д��ǿ������������
��2������ͼ��ʾ����NaOH�����ϵμ���Ũ��ˮ��Ѹ�ٸ��ϸǣ��۲�����
��Ũ����Һ�θ�������ְ��̣�������Ӧ�Ļ�ѧ����ʽΪ ��
��Ũ����Һ���Ϸ�û����������һ��ʱ���Ũ�����Һ�����а�ɫ���壬�ù�������� ��д��ѧʽ��һ�ּ��ɣ���
��FeSO4Һ�����ȳ��ֻ���ɫ��������һ��ʱ����ɺ��ɫ�������ķ�Ӧ����
Fe2++2NH3��H2O=Fe(OH)2��+ 2NH4+ �� ��
��3���������ѷ���Ŀǰ����NH3��ˮ����Ⱦ����Ҫ��������һ�������£���ˮ���м�������NaOH��ʹNH3���ѳ���������ƽ���ƶ�ԭ��������ԭ�� ��
��4�������������£���������ˮ�зֽ�����İ��ܹ��������������������ᣨHNO2������Ӧ�Ļ�ѧ����ʽΪ ������Ӧ����0.3 mol���ӷ���ת��ʱ�����������������Ϊ g��С���������λ��Ч���֣���
��1����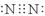 ��1�֣� ��ǿ��1�֣�
��1�֣� ��ǿ��1�֣�
��2����NH3+HCl=NH4Cl��1�֣� ��NH4HSO4��(NH4)2SO4��1�֣�
��4Fe(OH)2+O2+2H2O=4Fe(OH)3��2�֣�
��3������ˮ�д���ƽ�⣺NH3+H2O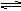 NH3��H2O
NH3��H2O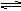 NH4+ +OH-��1�֣�д��NH3+H2O
NH4+ +OH-��1�֣�д��NH3+H2O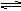 NH4+ +OH-���۷֣�������NaOH��OH-Ũ������ƽ�������ƶ���1�֣����������ڰ����ѳ���2�֣�
NH4+ +OH-���۷֣�������NaOH��OH-Ũ������ƽ�������ƶ���1�֣����������ڰ����ѳ���2�֣�
��4��2NH3+3O2 =2HNO2+2H2O��2�֣���д���ﲻ�۷֣� 2.35 g��2�֣�
=2HNO2+2H2O��2�֣���д���ﲻ�۷֣� 2.35 g��2�֣�
���������������1���ٵ���������Nԭ�Ӽ��γ�3�Թ��õ��ӣ�������Ϊ��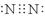
��NԪ�طǽ�����ǿ��PԪ�أ�����NH3���ȶ��Ա�PH3ǿ��
��2����Ũ����ӷ�����HCl��Ũ��ˮ�ӷ�����NH3��Ӧ�����ɵİ���ΪNH4Cl����ѧ����ʽΪ��NH3+HCl=NH4Cl��
��Ũ��ˮ�ӷ�����NH3������Ũ���������ᷴӦ���ɣ�NH4HSO4��(NH4)2SO4��
�����ձ�Ϊ���ɫ��������Fe(OH)3����ѧ����ʽΪ��4Fe(OH)2+O2+2H2O=4Fe(OH)3��
��3��NH3?H2OΪ������ڵ���ƽ�⣺NH3+H2O  NH3?H2O
NH3?H2O NH4++OH?������NaOH��������OH?Ũ�ȣ�ʹƽ�����淴Ӧ�����ƶ��������ڰ����ѳ���
NH4++OH?������NaOH��������OH?Ũ�ȣ�ʹƽ�����淴Ӧ�����ƶ��������ڰ����ѳ���
��4��NH3��O2��Ӧ�����������ˮ����ƽ�ɵû�ѧ����ʽ��2NH3+3O2 2HNO2+2H2O�����ݻ��ϼ۵ı仯��֪��6��n��HNO2��=0.3mol��n��HNO2��=0.05mol����m��HNO2��=2.35g��
2HNO2+2H2O�����ݻ��ϼ۵ı仯��֪��6��n��HNO2��=0.3mol��n��HNO2��=0.05mol����m��HNO2��=2.35g��
���㣺���⿼��������ͷ���ʽ����д��Ԫ�������ɡ�����ƽ�⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ1�ǹ�ҵ�ϡ���������� ʾ��ͼ����ͼ2�ǹ�ҵ�ϡ�ʯ�ҡ�ʯ����������ʾ��ͼ���ش�

��֪��
| �� �� | Ca(OH)2 | NaOH |
| �۸�Ԫ/kg�� | 0.36 | 2.9 |
| ����SO2�ijɱ���Ԫ/mol�� | 0.027 | 0.232 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ǿ������ʾ���Ũ���йء��ش��������⣺
��1��ijѧϰС����15 mol/LŨ��������100 mL3 mol/Lϡ���ᡣ
��������Ҫ����Ͳ��ȡ15 mol/LŨ����_______mL��
������ͼ��ʾ�����������ƹ����в����õ���_________������ţ���
��ͼ�����е������⣬����������Һ�����õ��IJ���������___________��
������ʵ������У�����ȷ����_________����д��ţ���
| A��ʹ������ƿǰ��������Ƿ�©ˮ�� |
| B������ʱҺ�泬���̶��ߣ������Һ��Ӧ�ý�ͷ�ι������� |
| C��������Һʱ������Ͳ��ȡŨ����ֱ�ӵ�������ƿ�У�Ȼ�������ˮ���ݡ� |
| D�����ݺ�Ǻ�ƿ����������ƿ�������µߵ���ҡ�ȡ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ᡢ��������������Ҫ�Ļ���ԭ��Ҳ�ǻ�ѧʵ������ر����Լ���
��1�������£��������۳�װ��Ũ���ᣬ������ΪŨ������� �ԣ�����Ӧ��������ɫ�Լ�ƿ�У�������Ϊ������� �ԣ����ڷ��õ�Ũ����Ũ�Ȼ��С��������Ϊ������� �ԡ�
��2������100 mL 18 mol��L��1��Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ�������������ڱ�״���µ����Ϊ13.44 L����μӷ�Ӧ��ͭƬ������Ϊ ��ѡ����ţ���
a��115.2 g b��76.8 g c��57.6 g d��38.4 g
����ʹ�������з�Ӧʣ���ͭƬ�����ܽ⣬�������м��������ƣ�д����Ӧ�����ӷ���ʽ�� ��
��3����ҵ���������Ե�ⱥ���Ȼ�����ҺΪ�������еģ��õ������������ĵ缫��ӦʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ��̽��������̬������(SO2��NO2��CO2)�����ʣ�ijͬѧ�����һ��ʵ�飺
ʵ��һ��̽������������ˮ�е��ܽ��ԣ�����֧��ͬ���Թ��ռ����������壬������ʢ��ˮ���ձ��У�һ��ʱ��۲쵽��������ͼA��B��C��ʾ��
��1������ͬ�����£�����������ˮ���ܽ��������________��д��ѧʽ��д��A�ձ��з�����Ӧ�Ļ�ѧ����ʽ��____ ���������ֻ�ձ��зֱ�μ�����ɫʯ����Һ���ɹ۲쵽��������_____________________ ��
ʵ���������ֻ����ƿ�ռ��������������������壬Ȼ���䵹����ˮ���С��ֱ���ͨ������O2��Cl2����ͼD��E��F��ʾ��һ��ʱ���D��Eװ�õļ���ƿ�г�����Һ��Fװ�õļ���ƿ�л�������ʣ�ࡣ
��2��ʵ�����װ��D�ļ���ƿ���ճ�����Һ(����ƿ��Һ�岻��ɢ)��
��д��װ��D���ܷ�Ӧ�Ļ�ѧ����ʽ��
_______________________________________________��
�ڼ����ʵ�������£�����Ħ�����Ϊa L��mol��1����װ��D�ļ���ƿ��������Һ���ʵ����ʵ���Ũ��Ϊ____________________��
��3�� д��ʵ��Fͨ������������Ӧ�Ļ�ѧ����ʽ��____________________________��
��4����Һ��������ƿ����Eװ�õ�ˮ����μ����ᱵ��Һ�����ܹ۲쵽������Ϊ________�����йص����ӷ���ʽ����ԭ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��G��X��Y������ѧ��ѧ�������ʣ�����B��D��E��F��G�ڳ�����Ϊ������BΪ����ɫ��C�Ǻ�ɫ�Ľ������ʡ�����֮��������ת����ϵ�����з�Ӧ�ۢܢߵIJ����е�ˮ����ȥ���������
��1��B�Ļ�ѧʽ________��
��2����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ ��
��3��д����Ӧ�ߵ����ӷ���ʽ_____________________��
��4��д�����X��Һ��������Ӧʽ______________________�������һ��ʱ���ת����0��2mol���ӣ����ʱ�������������ڱ�״���µ����Ϊ ��
��5��д����Ӧ�ܵĻ�ѧ����ʽ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧʵ����ͻ�����Ч�ؼ�����Ⱦ��ʵ�ֻ�ѧʵ����ɫ����Ҫ��ijѧ�������в�����һ��ʵ�飺��һ���³İ�ֽ�IJ���Ƭ�IJ�ͬλ�÷ֱ�μ�Ũ��Ϊ0.1 mol/L��KBr��KI(��������Һ)��NaOH(����̪)��FeCl2(��KSCN)��Һ��1�Σ�ÿ��Һ�α˴˷ֿ���Χ�ɰ뾶С�ڱ������Բ��(����ͼ��ʾ)����Բ��e������2��֥������С��KMnO4���壬��KMnO4�����ϵμ�һ��Ũ���ᣬ��������������Ǻá�(��֪��2KMnO4��16HCl(Ũ)=2KCl��2MnCl2��5Cl2����8H2O)
��1��e����Ӧ�����ӷ���ʽΪ_____________________���÷�Ӧ�з�����Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ ��
��2��b����ʵ������Ϊ________________��d����ʵ������Ϊ___________________��
��3��c����Ӧ�Ļ�ѧ����ʽΪ_______________________ ______����״���£�����0.224 L Cl2��NaOH��Һ���պ�ת�Ƶ��ӵ����ʵ���Ϊ mol��
��4��ͨ����ʵ���ܷ�Ƚ�Cl2��FeCl3��KMnO4�������������Ե�ǿ������������(��ܡ����ܡ�)�����ܣ�����������ǿ������˳���� ��(�����ܣ��˿ղ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������(NaC1O2)��һ����Ҫ�ĺ�������������Ҫ����ˮ�������Լ�ɰ�ǣ���֬��Ư����ɱ���������ǹ������ⷨ�����������ƵĹ�������ͼ��
��֪����NaC1O2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaC1O2��3H2O��
�ڴ�C1O2�ֽⱬը��һ����ϡ����������ϡ�͵�10%���°�ȫ��
I��������������̻ش���������
��1���������й�����������ÿ�����
��2���������ڵķ�Ӧ�Ļ�ѧ����ʽΪ �����������¶Ȳ��ܳ���20�棬��Ŀ���� ��
��3���ڼ�����Һ��NaC1O2�Ƚ��ȶ���������������Ӧά��NaOH�Թ������ж�NaOH�Ƿ�����ļ�ʵ�鷽���� ��
��4������Һ�еõ�NaC1O2��3H2O�־����ʵ����������� ��
II�����������е�ԭ��NaC1O3��������ͨ���ȵ��ռ���Һ���ؽᾧ�͵õ��Ƚϴ����IJ�Ʒ������ͨ�����������ȼ���Ʊ���
��1���ȼҵ�е�ԭ��Ϊ ��ԭ����Ҫ������ԭ���� ��������ƷΪ
��2����ҵ����NaC1O3�Ļ�ѧ����ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��12�֣����������������繤ҵ����ˮ����ķ������ʺϴӵ�Ũ�Ⱥ�����Һ����ȡ�塣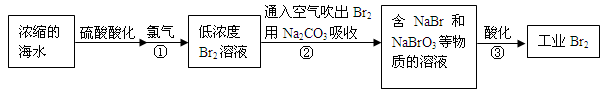
��1��NaBr�ĵ���ʽ�� ��
��2����Ӧ���ǽ�Br��ת��ΪBr2����Ӧ�ٵ����ӷ���ʽ�� ��
��3��ͨ���������Br2������Na2CO3���յ�Ŀ���� ��
��4����Ӧ�ڵĻ�ѧ����ʽ�� ��
��5����Ӧ����ÿ����3 mol Br2��ת�Ƶ��ӵ����ʵ����� mol��
��6��Ϊ�˳�ȥ��ҵBr2������Cl2������ҵBr2�� ������ĸ����
a��ͨ��HBrb������NaBr��Һ c������Na2CO3��Һ d������Na2SO3��Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com