
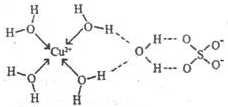
 ��������Ԫ�صĵ��ʼ��������ڹ�ũҵ������Ӧ�ù㷺��
��������Ԫ�صĵ��ʼ��������ڹ�ũҵ������Ӧ�ù㷺��| Ԫ�� | Mn | Fe | |
| ������kJ?mol-1 | I1 | 717 | 759 |
| I2 | 1509 | 1561 | |
| I3 | 3248 | 2957 | |
| 18-10 |
| 2 |


 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ɢ�����ӵ�ֱ����10-9��10-7m֮��ķ�ɢϵ�������� |
| B��KAl��SO4��2��ˮ��Һ�е��뷽��ʽΪKAl��SO4��2�TK++Al3++SO42- |
| C�����ö����ЧӦ���Լ���CuSO4��Һ�������������� |
| D����������������ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
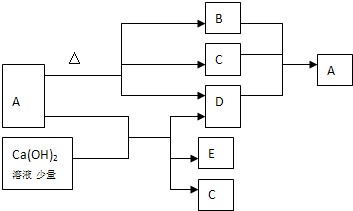
 A��B��C��D����ѧ��ѧ�ij������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת���Ĺ�ϵ����ͼ��ʾ�����ַ�Ӧ�е�H2 O����ȥ��������գ�
A��B��C��D����ѧ��ѧ�ij������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת���Ĺ�ϵ����ͼ��ʾ�����ַ�Ӧ�е�H2 O����ȥ��������գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
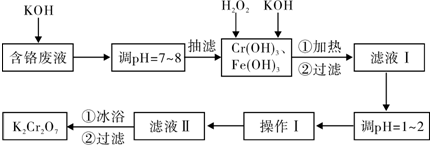
| ���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
| KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
| K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 |
| K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
| KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
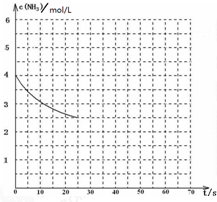 ��������Ҫ�Ļ�����Ʒ֮һ��
��������Ҫ�Ļ�����Ʒ֮һ��| 1 |
| 2 |
| 1 |
| 2 |
| ������� | H2CO3 | NH3?H2O |
| ����ƽ�ⳣ�� | Ka1=4.30��10-7 Ka2=5.61��10-11 | Kb=1.77��10-5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| CH4 | SiH4 | NH3 | PH3 | |
| �е㣨K�� | 101.7 | 161.2 | 239.7 | 185.4 |
| �ֽ��¶ȣ�K�� | 873 | 773 | 1073 | 713.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1L 1.0mol?L-1��NaC1O��Һ�к���C1O-����ĿΪNA |
| B����״���£�22.4L���Ậ��NA��HC1���� |
| C�����³�ѹ�£�14g��N2��CO��ɵĻ�����庬�е�ԭ����ĿΪNA |
| D��1molNa�������������Na2O2��ʧȥ��2NA���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com