��2011?̩��һģ�������ͣ�������Ὣ�����⾭��ʱ����Ŀǰ���ģ���ⷽʽ���ǻ�ѧ���⣮
I������������
�Լ״�Ϊ������Ӧ��������һ��;���У�CH
3OH��l��=2H
2��g��+CO��g������H
1=+128kJ?mol
-1CH
3OH��l��+H
2O��l��=3H
2��g��+CO
2��g������H
2=a kJ?mol
-1��֪��H
2��g��+1/2O
2��g��=H
2O��l������H=-286kJ?mol
-1Ϊ��á�H
2����Ҫ֪��
CO
CO
��ȼ���ȣ�����ȼ����Ϊ��H=һ283kJ?mol
-1�����H
2=
+131kJ/mol
+131kJ/mol
��
�����û�����
��1���о����������и�Ľ���������кܸߵķ�Ӧ���ԣ����������Ͻ���ˮ�б��и�������ʱ���Գ������ͷų�������ʹ������ˮ��Ӧ��������ʹ���������ý�����ˮ��Ӧ���������ŵ��У��ټ������ɱ��ϵͣ���
����е�и���Ϊֹͣʱ�����ⷴӦҲ������ֹͣ
����е�и���Ϊֹͣʱ�����ⷴӦҲ������ֹͣ
��
��2��������Ȼ�������õ���CO��H
2������Խ�����������л�ԭ��Ȼ������ˮ��Ӧ�ų��������ɴ˴��һ������ѭ�������ݸ�����ѧ��֪ʶ����Ԥ��ý������ʣ���д���ý����ڼ��ȵ���������ˮ������Ӧ�Ļ�ѧ����ʽ��
3Fe+4H
2O��g��
Fe
3O
4+4H
2��
3Fe+4H
2O��g��
Fe
3O
4+4H
2��
��
III��̫�������⣺���ù��ֽܷ�ˮ����Ҫ�д����IJ��룮�����йش�����˵����ȷ����
CD
CD
A��ʹ�ø�Ч�����ֽ�ˮ�Ƶ�H
2��ͬʱ�����Ի������
B��ʹ�ø�Ч����������ˮ�����Է��ֽ�
C���轺���ж�ṹ���нϴ�ı����������������������
D�����ڿ��淴Ӧ����������������Ӧ���ʵ�ͬʱҲ�����淴Ӧ����
������λ�⻯������
�����⻯�ƣ�NaBH
4��ˮ��Һ�м����ض���������Ѹ�ٵط���ˮ�ⷴӦ����ƫ�����ƺ���������д����ˮ�ⷴӦ�Ļ�ѧ����ʽ��
NaBH4+2H2O=NaBO2+4H2��
NaBH4+2H2O=NaBO2+4H2��
��
����ˮƫ�����ơ��⻯þ��MgH
2��������ĥ�豸�У�ͨ�����������������ѹǿ100��500kPa��ĥ0.5��4h�����ɵõ����⻯�ƣ���ĥ��������Ҫͨ�����������������ѹǿ100��500kPa��Ŀ���ǣ�
��ֹNaBH4����������ֹ�����⻯��ˮ��
��ֹNaBH4����������ֹ�����⻯��ˮ��
��

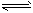 2HI(g) ��H < 0������ͬ�ݻ��Ķ����ܱ��������ң����м���H2��I2��0.1mol�����м���HI 0.2mol����ͬ�¶��·ֱ�ﵽƽ�⡣��ʹ����HI��ƽ��Ũ�ȴ�������HI��ƽ��Ũ�ȣ�Ӧ��ȡ�Ĵ�ʩ��
2HI(g) ��H < 0������ͬ�ݻ��Ķ����ܱ��������ң����м���H2��I2��0.1mol�����м���HI 0.2mol����ͬ�¶��·ֱ�ﵽƽ�⡣��ʹ����HI��ƽ��Ũ�ȴ�������HI��ƽ��Ũ�ȣ�Ӧ��ȡ�Ĵ�ʩ�� 
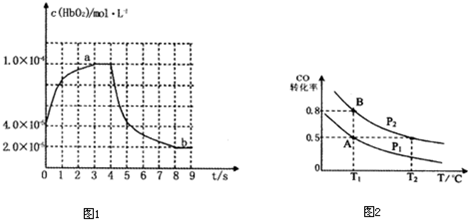
 ͨ��ú��������Һ����ʹ̼���仯������Թ㷺Ӧ�ã�
ͨ��ú��������Һ����ʹ̼���仯������Թ㷺Ӧ�ã�