
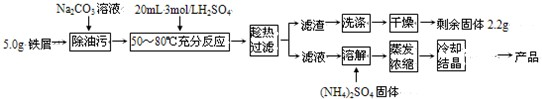
 ��������茶���[��NH4��2Fe��SO4��2?6H2O]�ڿ����б�һ���������ȶ����Ƿ�����ѧ�г����Ļ�ԭ������ʵ�����Ʊ��������£�
��������茶���[��NH4��2Fe��SO4��2?6H2O]�ڿ����б�һ���������ȶ����Ƿ�����ѧ�г����Ļ�ԭ������ʵ�����Ʊ��������£� HCO3-+OH-��ʹNa2CO3��Һ�ʼ��ԣ����Լ��Ե�Na2CO3��Һϴȥ�������ۣ�
HCO3-+OH-��ʹNa2CO3��Һ�ʼ��ԣ����Լ��Ե�Na2CO3��Һϴȥ�������ۣ� HCO3-+OH-��
HCO3-+OH-��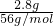 =0.05mol������������茶���[��NH4��2Fe��SO4��2?6H2O]��֪����Ҫ0.05mol��NH4��2SO4�����Գ�����NH4��2SO4��������Ϊ0.05mol��132g/mol=6.6g��
=0.05mol������������茶���[��NH4��2Fe��SO4��2?6H2O]��֪����Ҫ0.05mol��NH4��2SO4�����Գ�����NH4��2SO4��������Ϊ0.05mol��132g/mol=6.6g��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ϻ����ɽ���������ѧ����ĩ��һģ�����Ի�ѧ�Ծ��������棩 ���ͣ������
�̷���FeSO4��7H2O���ڻ�ѧ�ϳ���������ԭ������������ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷���
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ ����>��<��=��40%��
��2��ʵ��������20%�������ᣨ100�˷������ẬSO3 20�ˣ�����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL2mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS������������������λС������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com