
 B����CuO��CuO���ʵ���С��
B����CuO��CuO���ʵ���С��  mol
mol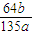 x100%
x100% ��A��ȷ��
��A��ȷ�� mol����B��ȷ��
mol����B��ȷ��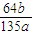 x100%����C����
x100%����C����

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| aNA |
| 80 |
| b |
| 135 |
| 64b |
| 135a |
�鿴�𰸺ͽ���>>
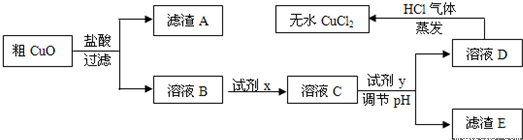
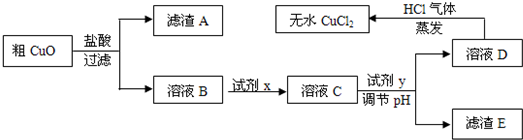
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ɶ����и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��ҵ�����Ȼ�ͭ��ˮ��ʱ���ǽ�Ũ����������������80 �����ң����������������ͭ��(����������������SiO2)����ֽ����ʹ֮�ܽ⣬��ȡ�������£� ��֪��pH��9.6ʱ��Fe2+��Fe(OH)2����ʽ��ȫ������pH��6.4ʱ��Cu2+��Cu(OH)2����ʽ��ȫ����������ʱKsp[Fe(OH)3]=c(Fe3+).c3(OH��)=1.0��10��38���Իش�
��֪��pH��9.6ʱ��Fe2+��Fe(OH)2����ʽ��ȫ������pH��6.4ʱ��Cu2+��Cu(OH)2����ʽ��ȫ����������ʱKsp[Fe(OH)3]=c(Fe3+).c3(OH��)=1.0��10��38���Իش�
(1)���ݳ���ʱFe(OH)3��Ksp���㣬����Һ�е�Fe3+��ȫ����(��ʱ��Һ�е�Fe3+���ʵ�����Ũ��=10��5mol/L)����Һ��pH= ��
(2)��ҺD��ȡ��ˮCuCl2ʱ������HCl���������ɣ�ԭ����
��
(3)��CuO���ڹ�������õ�����ҺB�м����Լ�x��Fe2+����ΪFe3+���Լ�x������ (������ѡ�����)��
A��KMnO4��Һ B����ˮ C��NaClO��Һ D��O3
����H2O2��Һ����Ӧ�����ӷ���ʽΪ ��
(4)��ҺC�����Լ�y��Ҫ������Һ��pH��3-4���Լ�y������ (������ѡ�����)��ԭ���� ��
A��NaOH��Һ B��Cu(OH)2���� C��Cu2(OH)2CO3���� D��CuO����
(5)��ag��CuO��������һϵ�в���֮�����յõ�bg��ˮCuCl2(������ʵ������в�����������)������˵����ȷ���� ��(NA��ʾ����٤������)
A����CuO��Cu2+��ĿС��
B����CuO��CuO���ʵ���С�� mol
mol
C����CuO��������ͭԪ�ص���������Ϊ ��100�G
��100�G
D����CuO�й���CuO������ȷ��
�鿴�𰸺ͽ���>>
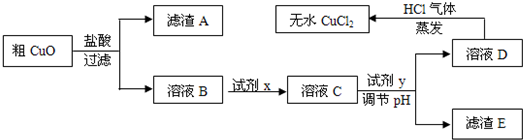
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��ҵ�����Ȼ�ͭ��ˮ��ʱ���ǽ�Ũ����������������80 �����ң����������������ͭ��(����������������SiO2)����ֽ����ʹ֮�ܽ⣬��ȡ�������£�
 ��֪��pH��9.6ʱ��Fe2+��Fe(OH)2����ʽ��ȫ������pH��6.4ʱ��Cu2+��Cu(OH)2����ʽ��ȫ����������ʱKsp[Fe(OH)3]=c(Fe3+).c3(OH��)=1.0��10��38���Իش�
��֪��pH��9.6ʱ��Fe2+��Fe(OH)2����ʽ��ȫ������pH��6.4ʱ��Cu2+��Cu(OH)2����ʽ��ȫ����������ʱKsp[Fe(OH)3]=c(Fe3+).c3(OH��)=1.0��10��38���Իش�
(1)���ݳ���ʱFe(OH)3��Ksp���㣬����Һ�е�Fe3+��ȫ����(��ʱ��Һ�е�Fe3+���ʵ�����Ũ��=10��5mol/L)����Һ��pH= ��
(2)��ҺD��ȡ��ˮCuCl2ʱ������HCl���������ɣ�ԭ����
��
(3)��CuO���ڹ�������õ�����ҺB�м����Լ�x��Fe2+����ΪFe3+���Լ�x������ (������ѡ�����)��
A��KMnO4��Һ B����ˮ C��NaClO��Һ D��O3
����H2O2��Һ����Ӧ�����ӷ���ʽΪ ��
(4)��ҺC�����Լ�y��Ҫ������Һ��pH��3-4���Լ�y������ (������ѡ�����)��ԭ���� ��
A��NaOH��Һ B��Cu(OH)2���� C��Cu2(OH)2CO3���� D��CuO����
(5)��ag��CuO��������һϵ�в���֮�����յõ�bg��ˮCuCl2(������ʵ������в�����������)������˵����ȷ���� ��(NA��ʾ����٤������)
A����CuO��Cu2+��ĿС��
B����CuO��CuO���ʵ���С�� mol
mol
C����CuO��������ͭԪ�ص���������Ϊ ��100�G
��100�G
D����CuO�й���CuO������ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| aNA |
| 80 |
| b |
| 135 |
| 64b |
| 135a |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com