
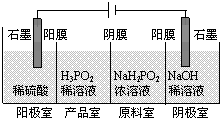
 “ĪĮ×Ėį£ØH3PO2£©ŹĒŅ»ÖÖ¾«Ļø»Æ¹¤²śĘ·£¬¾ßÓŠ½ĻĒ滹ŌŠŌ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
“ĪĮ×Ėį£ØH3PO2£©ŹĒŅ»ÖÖ¾«Ļø»Æ¹¤²śĘ·£¬¾ßÓŠ½ĻĒ滹ŌŠŌ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö £Ø1£©øł¾ŻH3PO2ŹĒŅ»ŌŖÖŠĒæĖįæÉÖŖ£¬H3PO2ŹĒČõµē½āÖŹ£¬ČÜŅŗÖŠ²æ·ÖµēĄė³öĒāĄė×Ó£¬¾Ż“ĖŠ“³öµēĄė·½³ĢŹ½£»
£Ø2£©¢Łøł¾Ż»ÆŗĻĪļÖŠ×Ü»ÆŗĻ¼ŪĪŖ0¼ĘĖć³öPŌŖĖŲµÄ»ÆŗĻ¼Ū£»
¢ŚĻČÅŠ¶ĻŃõ»Æ¼Į”¢Ńõ»Æ¼Į£¬Č»ŗóøł¾ŻŃõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ4£ŗ1¼ĘĖć³ö·“Ó¦²śĪļÖŠPµÄ»ÆŗĻ¼Ū£»
¢Ūøł¾ŻH3PO2ŹĒŅ»ŌŖÖŠĒæĖį£¬æÉŅŌÅŠ¶ĻNaH2PO2ĪŖÕżŃĪ£»
£Ø3£©øł¾Ż·“Ó¦ĪļŗĶÉś³ÉĪļ½įŗĻŌ×ÓŹŲŗćŗĶµē×ÓŹŲŗćŹéŠ“·½³ĢŹ½£»
£Ø4£©¢Łøł¾ŻŃō¼«ÖŠŅõĄė×ÓĪŖĮņĖįøłĄė×Ó”¢ĒāŃõøłĄė×ÓŗĶH2PO2-£¬ĒāŃõøłĄė×ÓŹ§µē×Ó·¢ÉśŃõ»Æ·“Ó¦£¬Ņõ¼«ÉĻŹĒĒāĄė×ӵƵ½µē×Ó·¢Éś»¹Ō·“Ó¦£¬Š“³öŅõ¼«µÄµē¼«·“Ó¦Ź½£»
¢Śøł¾ŻĶ¼Ź¾”°ĖÄŹŅµēÉųĪö·Ø”±¹¤×÷ŌĄķ·ÖĪö²śĘ·ŹŅæɵƵ½H3PO2µÄŌŅņ£»
¢Ūøł¾ŻH3PO2¼°NaH2PO2¾łČŻŅ×±»Ńõ»Æ·ÖĪöøĆ×°ÖĆȱµć£®
½ā“š ½ā£ŗ£Ø1£©H3PO2ŹĒŅ»ŌŖÖŠĒæĖį£¬ČÜŅŗÖŠ²æ·ÖµēĄė³öĒāĄė×Ó£¬ĖłŅŌĘäµēĄė·½³ĢŹ½ĪŖH3PO2?H2PO2-+H+£¬
¹Ź“š°øĪŖ£ŗH3PO2?H2PO2-+H+£»
£Ø2£©¢ŁH3PO2ÖŠ£¬×Ü»ÆŗĻ¼ŪĪŖ0£¬ĘäÖŠĒāŌŖĖŲĪŖ+1¼Ū£¬ŃõŌŖĖŲĪŖ-2¼Ū£¬ŌņPŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ+1¼Ū£¬
¹Ź“š°øĪŖ£ŗ+1£»
¢ŚøĆ·“Ó¦ÖŠAg+ĪŖŃõ»Æ¼Į£¬H3PO2ĪŖ»¹Ō¼Į£¬Ńõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ4£ŗ1£¬Éč·“Ó¦²śĪļÖŠPµÄ»ÆŗĻ¼ŪĪŖx£¬øł¾Ż»ÆŗĻ¼ŪÉż½µĻąµČæÉµĆ£¬4”Į£Ø1-0£©=1”Į£Øx-1£©£¬½āµĆx=5£¬ĖłŅŌŃõ»Æ²śĪļĪŖ+5¼ŪµÄH3PO4£¬
¹Ź“š°øĪŖ£ŗH3PO4£»
¢ŪÓÉÓŚH3PO2ŹĒŅ»ŌŖÖŠĒæĖį£¬ĖłŅŌNaH2PO2ĪŖÕżŃĪ£¬ÓÉÓŚH3PO2ŹĒŅ»ŌŖÖŠĒæĖį£¬ŌņNaH2PO2ĪŖĒæ¼īČõĖįŃĪ£¬ČÜŅŗ³ŹČõ¼īŠŌ£¬
¹Ź“š°øĪŖ£ŗÕżŃĪ£»Čõ¼īŠŌ£»
£Ø3£©°×Į×£ØP4£©ÓėBa£ØOH£©2ČÜŅŗ·“Ӧɜ³ÉPH3ĘųĢåŗĶBa£ØH2PO2£©2£¬·“Ó¦·½³ĢŹ½ĪŖ2P4+3Ba£ØOH£©2+6H2O=3Ba£ØH2PO2£©2+2PH3”ü£¬
¹Ź“š°øĪŖ£ŗ2P4+3Ba£ØOH£©2+6H2O=3Ba£ØH2PO2£©2+2PH3”ü£»
£Ø4£©¢ŁÓÉÓŚŃō¼«ÖŠŅõĄė×ÓĪŖĮņĖįøłĄė×Ó”¢ĒāŃõøłĄė×ÓŗĶH2PO2-£¬ĘäÖŠ·ÅµēÄÜĮ¦×īĒæµÄŹĒĒāŃõøłĄė×Ó£¬ŌņŃō¼«·¢ÉśµÄµē¼«·“Ó¦ĪŖ£ŗ4OH--4e-=O2”ü+2H2O£¬Ņõ¼«µē¼«·“Ó¦ŹĒČÜŅŗÖŠĒāĄė×ӵƵ½µē×Ó·¢Éś»¹Ō·“Ó¦£¬2H++2e-=H2”ü
¹Ź“š°øĪŖ£ŗ4OH--4e-=O2”ü+2H2O£»
¢Ś²śĘ·ŹŅæɵƵ½H3PO2µÄŌŅņŹĒŅņĪŖ£ŗŃō¼«ŹŅµÄH+“©¹żŃōĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬ŌĮĻŹŅµÄH2PO2-“©¹żŅõĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬¶žÕß·“Ӧɜ³ÉH3PO2£¬
¹Ź“š°øĪŖ£ŗŃō¼«ŹŅµÄH+“©¹żŃōĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬ŌĮĻŹŅµÄH2PO2-“©¹żŅõĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬¶žÕß·“Ӧɜ³ÉH3PO2£»
¢ŪŌēĘŚ²ÉÓĆ”°ČżŹŅµēÉųĪö·Ø”±ÖʱøH3PO2£¬½«”°ĖÄŹŅµēÉųĪö·Ø”±ÖŠŃō¼«ŹŅµÄĻ”ĮņĖįÓĆH3PO2Ļ”ČÜŅŗ“śĢę£¬²¢³·Č„Ńō¼«ŹŅÓė²śĘ·ŹŅÖ®¼äµÄŃōĤ£¬“Ó¶ųŗĻ²¢ĮĖŃō¼«ŹŅÓė²śĘ·ŹŅ£¬ĘäȱµćŹĒŃō¼«²śÉśµÄŃõĘų»į°ŃH2PO2-»ņH3PO2Ńõ»Æ³ÉPO43-£¬²śĘ·ÖŠ»ģÓŠPO43-£¬
¹Ź“š°øĪŖ£ŗPO43-£»H2PO2-»ņH3PO2±»Ńõ»Æ£®
µćĘĄ ±¾Ģāæ¼²é½ĻĪŖ×ŪŗĻ£¬Éę¼°µēĄė·½³ĢŹ½”¢»ÆŗĻ¼Ū”¢µē½āŌĄķ”¢ŃĪµÄĖ®½āŌĄķ”¢Ńõ»Æ»¹Ō·“Ó¦µČÖŖŹ¶£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·ŃĪµÄĖ®½ā”¢µē½āŌĄķĪŖ½ā“š¹Ų¼ü£¬ŹŌĢāÖŖŹ¶µć½Ļ¶ą”¢×ŪŗĻŠŌ½ĻĒ棬³ä·Öæ¼²éĮĖѧɜµÄ·ÖĪöÄÜĮ¦¼°Įé»īÓ¦ÓĆÄÜĮ¦£®


 Ė«»łĶ¬²½µ¼ŗ½ŃµĮ·ĻµĮŠ“š°ø
Ė«»łĶ¬²½µ¼ŗ½ŃµĮ·ĻµĮŠ“š°ø »ĘøŌŠ”דŌŖĶ¬²½¼ĘĖćĢģĢģĮ·ĻµĮŠ“š°ø
»ĘøŌŠ”דŌŖĶ¬²½¼ĘĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
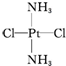 £¬»ĘĀĢÉ«¹ĢĢå
£¬»ĘĀĢÉ«¹ĢĢå £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | °±ĘųæÉÓĆÓŚÖĘŌģµŖ·Ź | B£® | ĀČĘųæÉÓĆÓŚĻū¶¾ŗĶÖĘŌģĘÆ°×·Ū | ||
| C£® | ÕįĢĒæÉŅŌŹ³ÓĆ | D£® | ÓĆ¹¤Ņµ¾Ę¾«¹“¶ŅµÄ°×¾ĘæÉŅŌŅūÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀĻĀ£¬ÓĆpH¼Ę²āĮæ0.1mol•L-1FeCl3ČÜŅŗµÄpHĪŖa£¬ŌŁ²āĮæĻ”ŹĶ10±¶µÄ0.01mol•L-1FeCl3ČÜŅŗµÄpHĪŖb£¬æÉÅŠ¶Ļ³öFeCl3Ė®½ā³Ģ¶ČÓėŃĪČÜŅŗÅØ¶Č“óŠ”¹ŲĻµ | |
| B£® | ½«×°ÓŠ3”«4mLĪŽĖ®ŅŅ“¼µÄŹŌ¹Ü½žČė50”ę×óÓŅµÄČČĖ®ÖŠ£¬½«ÉÕŗŚµÄĶĖæŃøĖŁ²åČėĪŽĖ®ŅŅ“¼ÖŠ£¬æɹŪ²ģµ½ĶĖæ±äŗģ£¬·“ø“Źż“Ī£¬æÉĪŵ½“Ģ¼¤ŠŌĘųĪ¶ | |
| C£® | ĻņijČÜŅŗ¼ÓÅØNaOHČÜŅŗ²¢Ī¢ČČ£¬Čō²śÉśµÄĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬ŌņĖµĆ÷“ĖČÜÖŹĪŖļ§ŃĪ | |
| D£® | Ėį¼īÖŠŗĶµĪ¶Øµ½ÖÕµć¶ĮŹżŹ±£¬·¢ĻÖµĪ¶Ø¹Ü¼ā×ģ“¦Šü¹ŅŅ»µĪ±ź×¼ČÜŅŗ£¬µ¼ÖĀŹµŃé½į¹ūĘ«øß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃéŠņŗÅ | ĘšŹ¼¶ĮŹż/mL | ÖÕµć¶ĮŹż/mL |
| I | 2.50 | 22.58 |
| ¢ņ | 1.00 | 23.12 |
| ¢ó | 0.00 | 19.92 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĻ©µÄ½į¹¹¼ņŹ½ĪŖCH2CH2 | B£® | ōĒ»łµÄµē×ÓŹ½£ŗ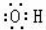 | ||
| C£® | Ca2+µÄ½į¹¹Ź¾ŅāĶ¼ĪŖ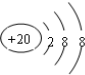 | D£® | ŅŅ“¼µÄ·Ö×ÓŹ½£ŗCH3CH2OH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
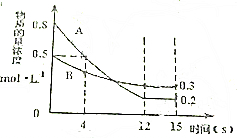 ŅŃÖŖ£ŗ·“Ó¦aA£Øg£©+bB£Øg£©?cC£Øg£©Ä³ĪĀ¶ČĻĀ£¬ŌŚ2LµÄĆܱÕČŻĘ÷ÖŠĶ¶ČėŅ»¶ØĮæµÄA”¢B£¬Į½ÖÖĘųĢåµÄĪļÖŹµÄĮæÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£®
ŅŃÖŖ£ŗ·“Ó¦aA£Øg£©+bB£Øg£©?cC£Øg£©Ä³ĪĀ¶ČĻĀ£¬ŌŚ2LµÄĆܱÕČŻĘ÷ÖŠĶ¶ČėŅ»¶ØĮæµÄA”¢B£¬Į½ÖÖĘųĢåµÄĪļÖŹµÄĮæÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ½Ī÷Ź”øßŅ»ÉĻµŚŅ»“ĪŌĀæ¼»Æѧ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
2015Äź11ŌĀ£¬Ņ»×éĆūĪŖĆ÷ŠĒ”°»ÆѧĖÄÉ·”±µÄÕÕʬ³ÉĪŖĮĖĶųÓŃĆĒČČŅéµÄ”°Ķ·ĢõŠĀĪÅ”±”£Ō±¾ŹĒĘÕĶصÄĖÄÕÅŠū“«ÕÕ»ņŹĒµēŹÓ¾ē¾µĶ·½ŲĶ¼£¬ÕÕʬ֊µÄĆ÷ŠĒ°Ś³öµÄ×ĖŹĘČ“¶¼ŗĶ»Æѧ²Ł×÷Ļą¹Ų”£µ«²»ĀŪŹĒĻØĆš¾Ę¾«µĘ»¹ŹĒŹ¹ÓĆĄäÄż¹Ü£¬Ć÷ŠĒĆĒ¶¼ÓĆæ“ĖĘøߥäµÄ·½Ź½£¬”°ĶźĆĄ”±±ÜæŖĮĖ»ÆѧŹµŃéÖŠµÄÕżČ·²Ł×÷£¬ČĆĶųÓŃĆĒČĢ攲»½ū”£ĻĀĮŠ»ł±¾ŹµŃé²Ł×÷ÕżČ·µÄŹĒ
A. Ļ”ŹĶÅØĮņĖįŹ±£¬½«Ė®ŃŲĘ÷±Ś»ŗĀż×¢ČėÅØĮņĖįÖŠ
B. ¹żĀĖŹ±£¬Ā©¶·ĄļŅŗĢåµÄŅŗĆęŅŖøßÓŚĀĖÖ½±ßŌµ
C. ½ŗĶ·µĪ¹ÜµÄ¹ÜæŚÖ±½ÓÉģČėŹŌ¹ÜĄļµĪ¼ÓŅŗĢ壬ŅŌĆāĶā½¦
D. ŹµŃéŹŅČ”ÓĆŅŗĢåŅ©Ę·×öŹµŃ鏱£¬Čē¹ūƻӊĖµĆ÷ÓĆĮ棬Ņ»°ćČ”1~2mL
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com