
���Ṥҵβ���еĵ���������(NO�� )����Ҫ�Ĵ�������䳣�õ�������������������(�Ѿ���)��
)����Ҫ�Ĵ�������䳣�õ�������������������(�Ѿ���)��
��NaOH���շ�����Ӧԭ�����£�
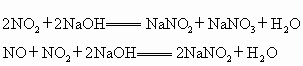
�ڰ�����ԭ������Ӧԭ���ɱ�ʾΪ
����һ�����ĺ� ��NO�����᳧β��(������������)�����ù�����NaOH��Һ���պ���Һ��
��NO�����᳧β��(������������)�����ù�����NaOH��Һ���պ���Һ�� ��
��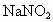 �����ʵ���֮��ǡ����β����NO��
�����ʵ���֮��ǡ����β����NO�� �����ʵ���֮����ȣ�
�����ʵ���֮����ȣ�
(1)���� ��ʾ��β���е����������ƽ����ɣ�����xֵ��
��ʾ��β���е����������ƽ����ɣ�����xֵ��
(2)��1����ĸ�β���ð�����ԭ���������������Ķ�������İ�(��״����)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
���Ṥҵβ���еĵ���������(NO��NO2)����Ҫ�Ĵ�����Ⱦ��֮һ���䳣�õ��������������¼��֣�
��NaOH��Һ���շ�����Ӧԭ�����£�
2NO2+2NaOH====NaNO2+NaNO3+H2O
NO2+NO+2NaOH====2NaNO2+H2O
�ڰ�����ԭ������Ӧԭ���ǣ�
NOx+NH3![]() N2+H2O
N2+H2O
����һ�����ĺ�NO2��NO��HNO3��ҵβ��(������������)�����ù�����NaOH��Һ���պ���Һ��NaNO3��NaNO2�����ʵ���֮��ǡ����β����NO��NO2�����ʵ���֮����ȡ�
(1)����NOx��ʾ��β���е����������ƽ����ɣ�����x��ֵ��
(2)��1����ĸ�β���ð�����ԭ���������������Ķ��������ͬ״���µİ���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
���Ṥҵβ���еĵ���������(NO��NO2)����Ҫ�Ĵ�����Ⱦ��֮һ���䳣�õ��������������¼��֣�
��NaOH��Һ���շ�����Ӧԭ�����£�
2NO2+2NaOH====NaNO2+NaNO3+H2O
NO2+NO+2NaOH====2NaNO2+H2O
�ڰ�����ԭ������Ӧԭ���ǣ�
![]()
![]()
![]()
����һ�����ĺ�NO2��NO��HNO3��ҵβ��(������������)�����ù�����NaOH��Һ���պ���Һ��NaNO3��NaNO2�����ʵ���֮��ǡ����β����NO��NO2�����ʵ���֮����ȡ�
(1)����NOx��ʾ��β���е����������ƽ����ɣ�����x��ֵ��
(2)��1����ĸ�β���ð�����ԭ���������������Ķ��������ͬ״���µİ���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
��NaOH��Һ���շ�����Ӧԭ�����£�
2NO2+2NaOH====NaNO2+NaNO3+H2O
NO2+NO+2NaOH====2NaNO2+H2O
�ڰ�����ԭ������Ӧԭ���ǣ�
NOx+NH3![]() N2+H2O
N2+H2O
����һ�����ĺ�NO2��NO��HNO3��ҵβ��(������������)�����ù�����NaOH��Һ���պ���Һ��NaNO3��NaNO2�����ʵ���֮��ǡ����β����NO��NO2�����ʵ���֮����ȡ�
(1)����NOx��ʾ��β���е����������ƽ����ɣ�����x��ֵ��
(2)��1����ĸ�β���ð�����ԭ���������������Ķ��������ͬ״���µİ���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ṥҵβ���еĵ���������(NO��NO2)����Ҫ�Ĵ�����Ⱦ��䳣�õ�������������������(�Ѽ�)
��NaOH���շ���Ӧԭ�����£�2 NO2+2NaOH=NaNO3+NaNO2+H2O��NO+NO2+2NaOH=2NaNO2+ H2O
�ڰ�����ԭ�� ��Ӧԭ���ǣ�NO2+NH3��N2+H2O
����һ�����ĺ�NO2��NO�����Ṥҵβ��(������������)�����ù�����NaOH��Һ���պ���Һ�� NaNO3�� NaNO2�����ʵ���֮��ǡ����β����NO�� NO2�����ʵ���֮����ȡ�
(1)����NOx��ʾ��β���е����������ƽ����ɣ�����x��ֵ��
(2)��1����ĸ�β���ð�����ԭ������������������ͬ״���¶�������İ���?
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com