
(��15��) �������ͼ����
��֪��һ��̼ԭ�������������ǻ�ʱ����������ת����
��1����Ӧ���������л���Ӧ������_______________��Ӧ��
��2����Ӧ�۵Ļ�ѧ����ʽ______________________________________________________��
��3����֪B����Է�������Ϊ162������ȫȼ�յIJ�����n(CO2)��n (H2O) =2��1����B�ķ���ʽΪ ��
��4��F�Ǹ߷��ӹ���������е���Ҫԭ�ϡ�F���������ص㣺���ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ���۱����ϵ�һ�ȴ���ֻ�����֡�F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������G��F��ͬ���칹�壬���ڷ����廯����ܷ���������Ӧ��G�Ľṹ�� �֡�
��6��������H��B��ͬ���칹�壬H�����к��еIJ��ֽṹΪ������ˮ����ᆳ�ۺϷ�Ӧ��ɵõ��߾���(CaHbO2)n.��H�ж��ֽṹ��д������һ�ֵĽṹ��ʽ ��
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�����15�֣���ע���������վ�ڵı�������ijЩʡ�м���վ�ṩ�����ͱ����90�š�93�Ż�����E90��E93���������������м�����10%��ȼ���Ҵ����Ƶõ��Ҵ����͡�
ȼ���Ҵ����������̿�����ͼ��ʾ��

��1����������Ŀ���ǣ� ��
��2������������Ϊ�˼������ˮ���Ƿ���ȫ����ʹ���Լ��� ��
��3������a�IJ����ǣ� ����
A������ B����ȡ C������ D����Һ
��4�����Ͳ�����CO2���ȿɴﵽ99%���ܻ������ã���ٳ�����������;�� ��
��5�������ȵ���ԭ�������Ҵ��Ļ�ѧ��Ӧ������ʽ��ʾ��
����������Ӧʽ���������100kg���������Ͽ�������ˮ�Ҵ� kg�������ԭ��������C-12 H-1 O-16��
��ĿǰΪֹ���ҹ������ϰٸ�����ʹ���Ҵ����͡�
��1��Ϊ�˱�������ȼ���Ҵ���ʳ���Ҵ�������ȼ���Ҵ�����������ú�ͣ�����������ú�͵����������ǣ� ��
��2����10%�Ҵ��������ڴ�������������Ӧ�ر�ע���ˮ�����������ˮ�������л���ֵ������� ��
��ͼ��ȼ���Ҵ������������ѹ���ʾ��ͼ����Ȼȼ���Ҵ���ʹ�û�����������Դ�Ľ���״�������Դ���һЩ���⡣�ɴ˿�֪��ȼ���Ҵ��� ��

A�������������ɫ��Դ
B���ṩ������������̫����
C�����������ѹ��̶Կ���û���κ�Ӱ��
D�����������н����Ĵ�����ʳ���Ա�����ʳ��ʣ
����ȼ���Ҵ��������ѣ�CH3OCH3���ͼ״���CH3OH��Ҳ����Ϊ����ȼ�ϣ��������Ҵ���Ϊͬ���칹����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ��ˮ�ж��и���ģ�⣨5�£����Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��1����ÿ��1�֣���1����3�֣������2�֣���15�֣�
ijУ��ѧ����С�������������ṩ������װ�ú�ҩƷ��ȡNaHCO3��Һ���������ʵ�顣ʵ�����ṩ����ҩƷ����2%NaOH��Һ��ϡHCl��ϡH2SO4�ܱ���KHCO3��Һ��ŨH2SO4��CaCO3�����K2CO3��ĩ�ിˮ���ṩ��������װ�ã�
�������ĿҪ��ش��������⣺
��l���밴�±�Ҫ����дѡ���װ�ú�ҩƷ
| ���� ���� | CO2����װ�ã�X�� �濪���ã������ͣ | ����ϴ��װ�ã�Y�� | �Ʊ���Ʒװ�ã�Z�� |
| ѡ���װ�ã�����ţ� | | | C |
| ѡ���ҩƷ������ţ� | | | �� |
 ����װ��(X)�������ԣ���д����Ҫ�������̣�
����װ��(X)�������ԣ���д����Ҫ�������̣� ���壬��ԭ����_________________��ͨ�����
���壬��ԭ����_________________��ͨ����� ��Zװ���ڵ���Һ������Ũ�ȴ�С˳��Ϊ��_______________________ ��
��Zװ���ڵ���Һ������Ũ�ȴ�С˳��Ϊ��_______________________ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ˮ�и���ģ�⣨5�£����Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��1����ÿ��1�֣���1����3�֣������2�֣���15�֣�
ijУ��ѧ����С�������������ṩ������װ�ú�ҩƷ��ȡNaHCO3��Һ���������ʵ�顣ʵ�����ṩ����ҩƷ����2%NaOH��Һ��ϡHCl��ϡH2SO4�ܱ���KHCO3��Һ��ŨH2SO4��CaCO3�����K2CO3��ĩ�ിˮ���ṩ��������װ�ã�

�������ĿҪ��ش��������⣺
��l���밴�±�Ҫ����дѡ���װ�ú�ҩƷ
|
���� ���� |
CO2����װ�ã�X�� �濪���ã������ͣ |
����ϴ��װ�ã�Y�� |
�Ʊ���Ʒװ�ã�Z�� |
|
ѡ���װ�ã�����ţ� |
|
|
C |
|
ѡ���ҩƷ������ţ� |
|
|
�� |
(2)��μ�����ѡ��� ����װ��(X)�������ԣ���д����Ҫ�������̣�
����װ��(X)�������ԣ���д����Ҫ�������̣�
_____________________________________________________________________________
__________________________________________________________________________��
(3)��װ�ð�X��Y��Z˳�����Ӳ���������Ժ�����ҩƷʵ��ʱ��Xװ���з�����ѧ��Ӧ�����ӷ���ʽΪ________________��Yװ���г�ȥ������Ϊ_____________��
(4)�����£���Zװ�õ�NaOH��Һ��ͨ����� ���壬��ԭ����_________________��ͨ�����
���壬��ԭ����_________________��ͨ����� ��Zװ���ڵ���Һ������Ũ�ȴ�С˳��Ϊ��_______________________
��
��Zװ���ڵ���Һ������Ũ�ȴ�С˳��Ϊ��_______________________
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������и����꼶5���¿������ۣ���ѧ���� ���ͣ������
(��15��) �������ͼ����
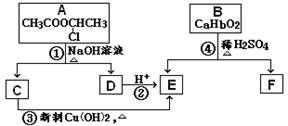
��֪��һ��̼ԭ�������������ǻ�ʱ����������ת����
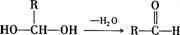
��1����Ӧ���������л���Ӧ������_______________��Ӧ��
��2����Ӧ�۵Ļ�ѧ����ʽ______________________________________________________��
��3����֪B����Է�������Ϊ162������ȫȼ�յIJ�����n(CO2)��n (H2O) = 2��1����B�ķ���ʽΪ ��
��4��F�Ǹ߷��ӹ���������е���Ҫԭ�ϡ�F���������ص㣺���ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ���۱����ϵ�һ�ȴ���ֻ�����֡�F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������G��F��ͬ���칹�壬���ڷ����廯����ܷ���������Ӧ��G�Ľṹ�� �֡�
��6��������H��B��ͬ���칹�壬H�����к��еIJ��ֽṹΪ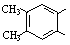 ������ˮ����ᆳ�ۺϷ�Ӧ��ɵõ��߾���(CaHbO2)n.��H�ж��ֽṹ��д������һ�ֵĽṹ��ʽ
��
������ˮ����ᆳ�ۺϷ�Ӧ��ɵõ��߾���(CaHbO2)n.��H�ж��ֽṹ��д������һ�ֵĽṹ��ʽ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com