
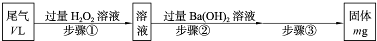
| O | 2-4 |
| mg |
| 233g/mol |
| m |
| 233 |
| m |
| 233 |
| 22.4m |
| 233 |
| ||
| VL |
| m��22.4 |
| 233V |
| m��22.4 |
| 233V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
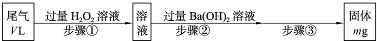
| O | 2- 4 |
| m��22.4 |
| 233V |
| m��22.4 |
| 233V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ����ר���̵�10�� �ǽ������仯������ϰ���������棩 ���ͣ������
SO2�����Ṥҵβ������Ҫ�ɷ֡�ʵ�����У�������ͼ��ʾ���̣��ⶨ��״�������ΪV L�����Ṥҵβ����SO2�ĺ�����
(1)�������м���H2O2��Һʱ������Ӧ�����ӷ���ʽΪ____________________��1 mol H2O2�μӷ�Ӧ��ת�Ƶĵ�����Ϊ________��
(2)�������IJ��������ǹ��ˡ�________��________�����ء�

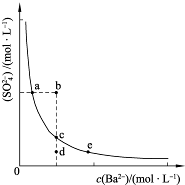
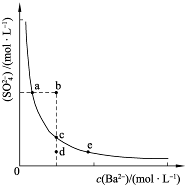
(3)һ���¶��£�BaSO4�ij����ܽ�ƽ��������ͼ��ʾ����������μ���Ba(OH)2��Һ�Ĺ����У�BaSO4���ܶȻ�����________(����������������С������������)����Һ��SO42-Ũ�ȵı仯���Ϊ________(�����)��
��d��c��e??????? ��b��c��d????? ��a��c��e???? ��d��c��a
(4)��V Lβ����SO2���������Ϊ________(�ú���V��m�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

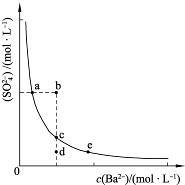
 Ũ�ȵı仯���Ϊ______������ţ�
Ũ�ȵı仯���Ϊ______������ţ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�����и߿���ѧ��ģ�Ծ��������棩 ���ͣ������

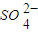 Ũ�ȵı仯���Ϊ______������ţ�
Ũ�ȵı仯���Ϊ______������ţ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com