
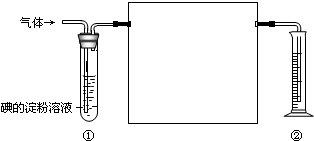
 һ��������к���SO2��N2��O2����ѧ��ȤС���ͬѧ���ⶨ����SO2�������������ͼ�������ڲⶨ��ʹ�õIJ���ʵ��װ�ã�
һ��������к���SO2��N2��O2����ѧ��ȤС���ͬѧ���ⶨ����SO2�������������ͼ�������ڲⶨ��ʹ�õIJ���ʵ��װ�ã� ��
�� ��
��| 0.0112L |
| 0.0112L+0.1488L |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
2- 4 |
- 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
+ 4 |
2- 3 |
2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.ij��Һ�м���BaCl2��Һ������ɫ����������ϡHNO3���ɫ�������ܣ�˵������Һ�����ٺ���Ag+��SO![]() �е�һ��
�е�һ��
B.��ȥNH3�е�����CO2���ɽ��������ͨ�������CaCl2����
C.N2�л��е�����NO2����ˮϴ��ȥ
D.NO�л�������NO2����ˮϴ��ȥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��������B�Ļ�ѧʽΪ_____________________��?
��2��A���Ƿ���NaCl_________����д���С���û�С�����������ʲô��ͨ������ش𣩣�
��3������Ӧ������1.008 L(��״��)һ�����壬�Ҹ����岻��ʹƷ����ɫ������ȡ��A��Ʒ�к���Na2S�����ʵ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com