
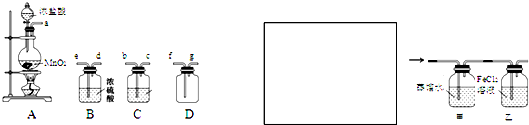
![]() ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅ”¢±ūČżøöÉÕ±ŅĄ“Ī·Ö±šŹ¢·Å100 g 5.00%µÄNaOHČÜŅŗ”¢×ćĮæµÄCuSO4ČÜŅŗŗĶ100 g 10.00%µÄK2SO4ČÜŅŗ£¬µē¼«¾łĪŖŹÆÄ«µē¼«”£
ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅ”¢±ūČżøöÉÕ±ŅĄ“Ī·Ö±šŹ¢·Å100 g 5.00%µÄNaOHČÜŅŗ”¢×ćĮæµÄCuSO4ČÜŅŗŗĶ100 g 10.00%µÄK2SO4ČÜŅŗ£¬µē¼«¾łĪŖŹÆÄ«µē¼«”£
![]()

![]() £Ø1£©½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆ±ūÖŠK2SO4ÅضČĪŖ10.47%£¬ŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£¾Ż“Ė»Ų“šĪŹĢā£ŗ
£Ø1£©½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆ±ūÖŠK2SO4ÅضČĪŖ10.47%£¬ŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£¾Ż“Ė»Ų“šĪŹĢā£ŗ
![]() ¢ŁµēŌ“µÄN¶ĖĪŖ£ß£ß£ß£ß£ß£ß¼«£»
¢ŁµēŌ“µÄN¶ĖĪŖ£ß£ß£ß£ß£ß£ß¼«£»
![]() ¢Śµē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
¢Śµē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
![]() ¢ŪĮŠŹ½¼ĘĖćµē¼«bÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż£ŗ
¢ŪĮŠŹ½¼ĘĖćµē¼«bÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż£ŗ
![]() £ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß
£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß
![]() ¢Üµē¼«cµÄÖŹĮæ±ä»ÆŹĒ£ß£ß£ß£ß£ß£ß£ß£ß£ßg£»
¢Üµē¼«cµÄÖŹĮæ±ä»ÆŹĒ£ß£ß£ß£ß£ß£ß£ß£ß£ßg£»
![]() ¢Żµē½āĒ°ŗóø÷ČÜŅŗµÄĖį”¢¼īŠŌ“󊔏Ē·ń·¢Éś±ä»Æ£¬¼ņŹöĘäŌŅņ£ŗ
¢Żµē½āĒ°ŗóø÷ČÜŅŗµÄĖį”¢¼īŠŌ“󊔏Ē·ń·¢Éś±ä»Æ£¬¼ņŹöĘäŌŅņ£ŗ
![]() ¼×ČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
¼×ČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
![]() ŅŅČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
ŅŅČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
![]() ±ūČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
±ūČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
![]() £Ø2£©Čē¹ūµē½ā¹ż³ĢÖŠĶČ«²æĪö³ö£¬“ĖŹ±µē½āÄÜ·ń¼ĢŠų½ųŠŠ£¬ĪŖŹ²Ć“£æ
£Ø2£©Čē¹ūµē½ā¹ż³ĢÖŠĶČ«²æĪö³ö£¬“ĖŹ±µē½āÄÜ·ń¼ĢŠų½ųŠŠ£¬ĪŖŹ²Ć“£æ
![]() £ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß”£
£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß”£
£Ø1£©¢ŁÕż¼« ¢Ś4OH£-4e£=2H2O + O2”ü”£¢Ū2.8L ¢Ü16g ¢Ż¼×Ōö“ó£¬ŅņĪŖĻąµ±ÓŚµē½āĖ®£»ŅŅ¼õŠ”£¬OH£·Åµē£¬ H£«Ōö¶ą”£±ū²»±ä£¬Ļąµ±ÓŚµē½āĖ®”£(2)æÉŅŌ
£Ø1£©¢ŁŅŅÖŠCµē¼«ÖŹĮæŌö¼Ó£¬Ōņc“¦·¢ÉśµÄ·“Ó¦ĪŖ£ŗCu2£«+2e£=Cu£¬¼“C“¦ĪŖŅõ¼«£¬ÓÉ“ĖæÉĶĘ³öbĪŖŃō¼«£¬aĪŖŅõ¼«£¬MĪŖøŗ¼«£¬NĪŖÕż¼«”£±ūÖŠĪŖK2SO4£¬Ļąµ±ÓŚµē½āĖ®£¬Éčµē½āµÄĖ®µÄÖŹĮæĪŖxg”£Óɵē½āĒ°ŗóČÜÖŹÖŹĮæĻąµČÓŠ£¬100”Į10%=£Ø100-x£©”Į10.47%£¬µĆx=4.5g£¬¹ŹĪŖ0.25mol”£ÓÉ·½³ĢŹ½2H2+O2 ![]() 2H2OæÉÖŖ£¬Éś³É2molH2O£¬×ŖŅĘ4molµē×Ó£¬ĖłŅŌÕūøö·“Ó¦ÖŠ×Ŗ»Æ0.5molµē×Ó£¬¶ųÕūøöµēĀ·ŹĒ“®ĮŖµÄ£¬¹ŹĆæøöÉÕ±ÖŠµÄµē¼«ÉĻ×ŖŅʵē×ÓŹżŹĒĻąµČµÄ”£¢Ś¼×ÖŠĪŖNaOH£¬Ļąµ±ÓŚµē½āH2O£¬Ńō¼«b“¦ĪŖŅõĄė×ÓOH£·Åµē£¬¼“4OH£-4e£=2H2O + O2”ü”£¢Ū×ŖŅĘ0.5molµē×Ó£¬ŌņÉś³ÉO2ĪŖ0.5/4=0.125mol£¬±źæöĻĀµÄĢå»żĪŖ0.125”Į22.4=2.8L”£¢ÜCu2£«+2e£=Cu£¬×ŖŅĘ0.5molµē×Ó£¬ŌņÉś³ÉµÄm(Cu)=0.5/2 ”Į64 =16g”£¢Ż¼×ÖŠĻąµ±ÓŚµē½āĖ®£¬¹ŹNaOHµÄÅضČŌö“ó£¬pH±ä“ó”£ŅŅÖŠŅõ¼«ĪŖCu2£«·Åµē£¬Ńō¼«ĪŖOH£·Åµē£¬ĖłŅŌH£«Ōö¶ą£¬¹ŹpH¼õŠ””£±ūÖŠĪŖµē½āĖ®£¬¶ŌÓŚK2SO4¶ųŃŌ£¬ĘäpH¼øŗõ²»±ä”££Ø2£©ĶČ«²æĪö³ö£¬æÉŅŌ¼ĢŠųµē½āH2SO4£¬ÓŠµē½āŅŗ¼“æɵē½ā”£
2H2OæÉÖŖ£¬Éś³É2molH2O£¬×ŖŅĘ4molµē×Ó£¬ĖłŅŌÕūøö·“Ó¦ÖŠ×Ŗ»Æ0.5molµē×Ó£¬¶ųÕūøöµēĀ·ŹĒ“®ĮŖµÄ£¬¹ŹĆæøöÉÕ±ÖŠµÄµē¼«ÉĻ×ŖŅʵē×ÓŹżŹĒĻąµČµÄ”£¢Ś¼×ÖŠĪŖNaOH£¬Ļąµ±ÓŚµē½āH2O£¬Ńō¼«b“¦ĪŖŅõĄė×ÓOH£·Åµē£¬¼“4OH£-4e£=2H2O + O2”ü”£¢Ū×ŖŅĘ0.5molµē×Ó£¬ŌņÉś³ÉO2ĪŖ0.5/4=0.125mol£¬±źæöĻĀµÄĢå»żĪŖ0.125”Į22.4=2.8L”£¢ÜCu2£«+2e£=Cu£¬×ŖŅĘ0.5molµē×Ó£¬ŌņÉś³ÉµÄm(Cu)=0.5/2 ”Į64 =16g”£¢Ż¼×ÖŠĻąµ±ÓŚµē½āĖ®£¬¹ŹNaOHµÄÅضČŌö“ó£¬pH±ä“ó”£ŅŅÖŠŅõ¼«ĪŖCu2£«·Åµē£¬Ńō¼«ĪŖOH£·Åµē£¬ĖłŅŌH£«Ōö¶ą£¬¹ŹpH¼õŠ””£±ūÖŠĪŖµē½āĖ®£¬¶ŌÓŚK2SO4¶ųŃŌ£¬ĘäpH¼øŗõ²»±ä”££Ø2£©ĶČ«²æĪö³ö£¬æÉŅŌ¼ĢŠųµē½āH2SO4£¬ÓŠµē½āŅŗ¼“æɵē½ā”£
![]()



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«Ź”¼ĆÄžŹŠ×Ž³Ē¶žÖŠø߶žÉĻѧʌʌ֊֏Įæ¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅĮ½øöÉÕ±·Ö±šŅĄ“ĪŹ¢·Å200mL±„ŗĶŹ³ŃĪĖ®”¢×ćĮæµÄAgNO3ČÜŅŗ£¬a”¢b”¢c”¢dĖÄøöµē¼«¾łĪŖŹÆÄ«µē¼«”£½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆŅŅÖŠdµē¼«ÖŹĮæŌö¼ÓĮĖ2.16g”£¾Ż“Ė»Ų“šĪŹĢā£ŗ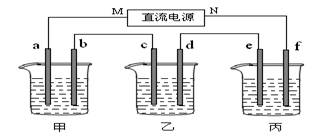
£Ø1£©µēŌ“µÄN¶ĖĪŖ ¼«£»
£Ø2£©µē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £»
£Ø3£©µē¼«cÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“Ģ¬ĻĀµÄĢå»ż£ŗ £»
£Ø4£©¼×ČÜŅŗµÄĒāŃõøłĄė×ÓÅضČĪŖ £ØÉčČÜŅŗĢå»żČŌĪŖ200mL)£»
£Ø5£©ÓūŌŚ±ūÉÕ±ÖŠŹµĻÖĢśµÄ±ķĆę¶ĘÉĻŅ»²ćĶ£¬Ōņµē½āÖŹČÜŅŗĪŖ £¬eµē¼«µÄ²ÄĮĻŹĒ£ŗ £¬fµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÖŲĒģŹŠĶņÖŻ¶žÖŠøßČżĒļ¼¾æŖѧ²āŹŌ»Æѧ£ØĄķ£©ŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©A”¢B”¢CČżÖÖĒæµē½āÖŹ£¬ĖüĆĒŌŚĖ®ÖŠµēĄė³öµÄĄė×ÓČēĻĀ±ķĖłŹ¾£ŗ
| ŃōĄė×Ó | Na+”¢K+”¢Cu2+ |
| ŅõĄė×Ó | SO42”Ŗ”¢OH£ |


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ½Ī÷Ź”°×šŲ֎֊ѧø߶žĻĀѧʌµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅ”¢±ūČżøöÉÕ±ŅĄ“Ī·Ö±šŹ¢·Å100 g 5.00%µÄNaOHČÜŅŗ”¢×ćĮæµÄCuSO4ČÜŅŗŗĶ100 g 10.00%µÄK2SO4ČÜŅŗ£¬µē¼«¾łĪŖŹÆÄ«µē¼«”£


 £Ø1£©½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆ±ūÖŠK2SO4ÅضČĪŖ10.47%£¬ŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£
£Ø1£©½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆ±ūÖŠK2SO4ÅضČĪŖ10.47%£¬ŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£
¾Ż“Ė»Ų“šĪŹĢā£ŗ ¢ŁµēŌ“µÄN¶ĖĪŖ ¼«£»
¢ŁµēŌ“µÄN¶ĖĪŖ ¼«£» ¢Śµē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £»
¢Śµē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £» ¢Ūµē¼«bÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż£ŗ
¢Ūµē¼«bÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż£ŗ
 ¢Üµē¼«cµÄÖŹĮæ±ä»ÆŹĒ g£»
¢Üµē¼«cµÄÖŹĮæ±ä»ÆŹĒ g£» £Ø2£©Čē¹ūµē½ā¹ż³ĢÖŠĶČ«²æĪö³ö£¬“ĖŹ±µē½āÄÜ·ń¼ĢŠų½ųŠŠ£¬ĪŖŹ²Ć“£æ
£Ø2£©Čē¹ūµē½ā¹ż³ĢÖŠĶČ«²æĪö³ö£¬“ĖŹ±µē½āÄÜ·ń¼ĢŠų½ųŠŠ£¬ĪŖŹ²Ć“£æ  ”£
”£ ¢Żµē½āĒ°ŗóø÷ČÜŅŗµÄpHČēŗĪ±ä»Æ”££ØĢīŌö“󣬼õŠ”»ņ²»±ä£©
¢Żµē½āĒ°ŗóø÷ČÜŅŗµÄpHČēŗĪ±ä»Æ”££ØĢīŌö“󣬼õŠ”»ņ²»±ä£© ¼×ČÜŅŗ £»
¼×ČÜŅŗ £» ŅŅČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£»
ŅŅČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£» ±ūČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£»
±ūČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģɽ¶«Ź”¼ĆÄžŹŠø߶žÉĻѧʌʌ֊֏Įæ¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅĮ½øöÉÕ±·Ö±šŅĄ“ĪŹ¢·Å200mL±„ŗĶŹ³ŃĪĖ®”¢×ćĮæµÄAgNO3ČÜŅŗ£¬a”¢b”¢c”¢dĖÄøöµē¼«¾łĪŖŹÆÄ«µē¼«”£½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆŅŅÖŠdµē¼«ÖŹĮæŌö¼ÓĮĖ2.16g”£¾Ż“Ė»Ų“šĪŹĢā£ŗ

£Ø1£©µēŌ“µÄN¶ĖĪŖ ¼«£»
£Ø2£©µē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £»
£Ø3£©µē¼«cÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“Ģ¬ĻĀµÄĢå»ż£ŗ £»
£Ø4£©¼×ČÜŅŗµÄĒāŃõøłĄė×ÓÅضČĪŖ £ØÉčČÜŅŗĢå»żČŌĪŖ200mL)£»
£Ø5£©ÓūŌŚ±ūÉÕ±ÖŠŹµĻÖĢśµÄ±ķĆę¶ĘÉĻŅ»²ćĶ£¬Ōņµē½āÖŹČÜŅŗĪŖ £¬eµē¼«µÄ²ÄĮĻŹĒ£ŗ £¬fµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com