
| ʵ����� | FeSO4��Һ���������/mL | |
| �ζ�ǰ | �ζ��� | |
| 1 | 0.10 | 16.20 |
| 2 | 0.30 | 15.31 |
| 3 | 0.20 | 15.19 |
| 1.56��10-10 |
| 3 | 2��1.10��10 -12 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.ʵ�����Ʊ���ϩʱ���뽫�¶ȼ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ�¶�
B.ȡ����������Һ��ϡ����ˮԡ���ȼ����Ӻ�����Ƶ�������ͭ��Һ���ȣ��۲������ж�����ˮ��IJ������Ƿ���������
C.�ڱ��м�����ˮ��������ã��۲������жϱ������Ƿ����̼̼˫��
D.ʵ�����Ʊ���������ʱ���Ƚ��Ҵ��������ϣ��ٰѻ��Һ���뵽Ũ������
E.��ȥ��������Һ��������NaCl���ɽ�װ�л��Һ�İ�Ĥ��������ˮ������
F.�к͵ζ�ʱ����ʽ�ζ���������ˮ��ϴ���κ������������еζ�
G.ʵ�����ýྻ���Թ���������Ӧʱ���ܽ��Թ�ֱ�ӷ��ھƾ��ƻ����ϼ���
��.��������̼���о��ɹ������˿Ƽ��Ľ��������õ绡���ϳɵ�̼���ܳ����д��������ʡ���̼������������̼���������������������ᴿ���䷴ӦʽΪ��
![]()
![]()
��1���˷�Ӧ����������______________������������______________��
��2��H2SO4��������Ӧ�б��ֳ�����������______________����ѡ���ţ���
A.���� B.������ C.��ˮ�� D.��ˮ��
��3����ƽ�Ļ�ѧ������Ϊ_________________��
��4��������Ӧ��������0.1 mol CO2���壬��ת�Ƶ��ӵ����ʵ�����____________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(14��)��������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��
��1��ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ
���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ���� ��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������
��װ��A�����ܽ�����Ķ����������壬�������ʢ�� (����ĸ)��
A��20mL 60�����ˮ B��100mL��ˮ
C��100mL����ʳ��ˮ D��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ
��2����ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������()������ˮ��ClO2��
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:
������4�м����ָʾ��Ϊ ���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
�۲���5��Ŀ����ʹ����Һ�е�
��ԭΪ
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
��������ˮ���ĺ������꣬�������м���������
��
��ԭΪ
����÷�Ӧ����������Ϊ (�ѧʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
��16�֣���������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��
(1)ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ

 ��
��
���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ����_ __��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������_____________��

��װ��A�����ܽ�����Ķ����������壬�������ʢ��_______(����ĸ)��
a��20mL 60�����ˮ b��100mL��ˮ
c��100mL����ʳ��ˮ d��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ______________________
(2)��ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������( )������ˮ��ClO2��
)������ˮ��ClO2�� �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������____________________________��
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:

 ������4�м����ָʾ��Ϊ_________���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ___________________________��
������4�м����ָʾ��Ϊ_________���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ___________________________��
�۲���5��Ŀ����ʹ ����Һ�е�
����Һ�е� ��ԭΪ
��ԭΪ �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ_________________
_____________��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ_________________
_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�ƺ����߸߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�ʵ����
(14��)��������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��
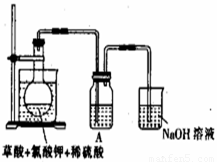
��1��ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ
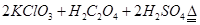
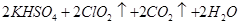
���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ���� ��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������
��װ��A�����ܽ�����Ķ����������壬�������ʢ�� (����ĸ)��
A��20mL 60�����ˮ B��100mL��ˮ
C��100mL����ʳ��ˮ D��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ
��2����ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������(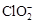 )������ˮ��ClO2��
)������ˮ��ClO2��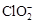 �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:

 ������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
�۲���5��Ŀ����ʹ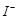 ����Һ�е�
����Һ�е�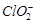 ��ԭΪ
��ԭΪ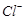 �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
��������ˮ��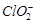 �ĺ������꣬�������м���������
�ĺ������꣬�������м���������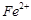 ��
��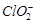 ��ԭΪ
��ԭΪ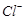 ����÷�Ӧ����������Ϊ
(�ѧʽ)
����÷�Ӧ����������Ϊ
(�ѧʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10������һ�и߶���ѧ�ڵڶ��ζο���ѧ�� ���ͣ�ʵ����
(14��)��������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��

(1)ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ


���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ���� ��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������
��װ��A�����ܽ�����Ķ����������壬�������ʢ�� (����ĸ)��
A��20mL 60�����ˮ B��100mL��ˮ
C��100mL����ʳ��ˮ D��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ
(2)��ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������(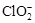 )������ˮ��ClO2��
)������ˮ��ClO2��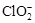 �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:

 ������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
�۲���5��Ŀ����ʹ ����Һ�е�
����Һ�е� ��ԭΪ
��ԭΪ �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
��������ˮ�� �ĺ������꣬�������м���������
�ĺ������꣬�������м��������� ��
�� ��ԭΪ
��ԭΪ ����÷�Ӧ����������Ϊ
(�ѧʽ)
����÷�Ӧ����������Ϊ
(�ѧʽ)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com