
��֪X��M������ѧ�̲ij���Ԫ��,���ж��������ӷ�Ӧͨʽ���ƶ���,������ȷ����( )
(��) XO3n-+Xn- + H+ ��X����+ H2O(δ��ƽ); (��)Mm++mOH-��M(OH)m��
����n=1,��XO3n-��XԪ��Ϊ+5��,Xλ�����ڱ��ڢ�A��
����n=2,��X����������ˮ��������������⻯�ﷴӦ
����m=1,��M��NO3��m��Һ�Ͱ�ˮ����ʱ��������ܲ�ͬ
����m=2,���ڿ��������ɡ�����MSO4��Һһ���ܵõ�MSO4
����m=3,��MCl3����������������Һ��Ӧһ������M��OH��m
A���٢� B�� �ڢ� C�� �٢� D�� �ܢ�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ұ��ҵ�ϣ���������ͨ����ѧ��ԭ���ƵõĽ������� (����)
A��Na��Mg ��Al�� B��Na��K ��Zn ��
C��Fe��Cu��Ag D��Na��Ca��K
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʳ�������������в���ȱ�ٵ����ʣ���ˮ�к��д���ʳ�Ρ�
ij�س����Ĵ����У�����������CaCl2��ͨ�������ʵ����Ƶô�����NaCl2��
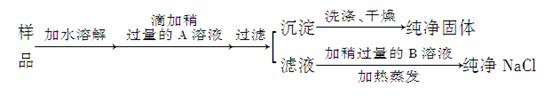 ��ش�
��ش�
(1)�����A��__________������A�ѹ����ķ�����_________________________
________________________________________________________________________��
(2)�����B��__________�������Թ���B��Ŀ����________________________��
(3)Ϊ��������Ƿ�ϴ������������ϴ��Һ�м���______________________��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���õ��¼����ɴ��������еĵ�������ں����ܱ������з������л�ѧ��Ӧ��
4NH3(g)��6NO(g) 5N2(g)��6H2O(l)
5N2(g)��6H2O(l)
��H��Q kJ/mol(Q<0)�������й�˵����ȷ���� (����)��
A�������������䣬ʹ�ø�Ч�����������е��������ת��������
B��ƽ��ʱ�������������䣬����NH3��Ũ�ȣ�������NO��ת���ʼ�С
C����λʱ��������NH3��H2O�����ʵ���֮��Ϊ2��3ʱ����Ӧ�ﵽƽ��
D��ƽ��ʱ�������������䣬�����¶ȿ�ʹ�÷�Ӧ��ƽ�ⳣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�ϳ�������Ҫ�ɷ���һ����̼�������������ںϳɼ��ѵ����ȼ�ϡ�����Ȼ����øúϳ��������п��ܷ����ķ�Ӧ�У�
��CH4(g)��H2O(g)CO(g)��3H2(g) ��H1����206.1 kJ��mol��1
��CH4(g)��CO2(g)2CO(g)��2H2(g) ��H2����247.3 kJ��mol��1
��CO(g)��H2O(g)CO2(g)��H2(g)����H3
��ش��������⣺
(1)��һ�ܱ������н��з�Ӧ�٣����CH4�����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ1��ʾ��

ͼ1
��Ӧ���е�ǰ5 min�ڣ�v(H2)��________��10 minʱ���ı���������������______________________________________________________________��
(2)��ͼ2��ʾ���ڼס����������зֱ��������ʵ�����CH4��CO2��ʹ�ס�����������ʼ�ݻ���ȡ�����ͬ�¶��·�����Ӧ�ڣ���ά�ַ�Ӧ�������¶Ȳ��䡣��֪��������CH4��ת������ʱ��ı仯��ͼ3��ʾ������ͼ3�л�����������CH4��ת������ʱ��仯��ͼ��

(3)��Ӧ���Ц�H3��________��800 ��ʱ����Ӧ�۵�ƽ�ⳣ��K��1����ø��¶����ܱ�������ijʱ�̸����ʵ����ʵ������±���
| CO | H2O | CO2 | H2 |
| 0.5 mol | 8.5 mol | 2.0 mol | 2.0 mol |
��ʱ��Ӧ���������淴Ӧ���ʵĹ�ϵʽ��________(�����)��
a��v��>v���� B��v��<v��
c��v����v���� D�����ж�
(4)�øúϳ�����ȡ���ѵĻ�ѧ����ʽΪ______________________________��
�÷�Ӧ��ԭ��������Ϊ________(�跴Ӧ��ȫ���У��������ٷֱȱ�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ���Ʊ��������װ�ã���Ӧ��Ҫ
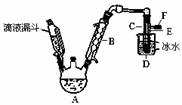 ���ȣ�ͼ��ʡȥ�˼���װ�á��й����ݼ�����
���ȣ�ͼ��ʡȥ�˼���װ�á��й����ݼ�����
�����Ҵ��������顢���йز���
| �Ҵ� | ������ | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | �����ɫҺ�� |
| �ܶ�/g•cm��3 | 0.79 | 1.44 | 3.1 |
| �е�/�� | 78.5 | 38.4 | 59 |
�� �Ʊ������У������Ũ����������ϡ�ͣ���Ŀ���� ��ѡ����ţ���
a�����ٸ�����ϩ���ѵ����� b������Br2������ c������HBr�Ļӷ�d��ˮ�Ƿ�Ӧ�Ĵ���
�� ��֪�����¶Ƚϵ�ʱNaBr�����ᷴӦ����NaHSO4��д������ʱA�з�������Ҫ��Ӧ
�Ļ�ѧ����ʽ ��
�� ����B������ ����ȴˮӦ��B�� ����ϡ����¡�����������
�� ��Ӧ���ɵ�������Ӧ�� �У��A����C���У���
�� ����Ũ���������ʵ��ʱ���õ�����������ػ�ɫ�����ѡ������ ��ѡ����ţ�
��Һ��ϴ�Ӳ�Ʒ��
a���������� b���������� c���⻯���� d��̼������
ϴ�Ӳ�Ʒʱ����Ҫ�IJ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���pH�仯���ж���ȷ����(����)
A���¶�����Na2CO3��ҺpH��С
B���¶����ߣ���ˮpH����
C��������ˮ������һ��ʱ�����ҺpH��С
D������������Һ�����ڿ����У���ҺpH���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��2.00g C2H2��ȫȼ������Һ̬ˮ��CO2���ų�99.6kJ��������д����ʾC2H2ȼ���ȵ��Ȼ�ѧ����ʽ��
��2��C2H2����������ȼ�ϵ�أ���д��KOH��ҺΪ�������Һ����Ȳȼ�ϵ�صĸ�����Ӧʽ��
��3������Ȳȼ�ϵ�ص��AgNO3��Һ����ʯī��������������д�����Դ����������ʯī�缫�ĵ缫��Ӧʽ��
��4�����������һ�����Ϳɳ���أ�����ͨ���ܵ����ȣ��õ���ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��
3Zn + 2K2FeO4 + 8H2O  3Zn(OH)2 + 2Fe(OH)3 + 4KOH
3Zn(OH)2 + 2Fe(OH)3 + 4KOH
��д���õ���ڳ��ʱ�����ĵ缫��Ӧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F��G��L��I��������Ԫ�طֲ���������ͬ�Ķ����ڣ����ǵ�ԭ������������������B��C��DΪͬһ���ڣ�A��E��B��G��D��L�ֱ�Ϊͬһ���壬C��D��F����Ԫ�ص�ԭ������֮��Ϊ28��F����������D��5��D��������������F��2����C��D������������֮��Ϊ11����ش��������⣺
��1�����Ϸǽ���Ԫ�������γɵ������̬�⻯���ȶ����������ǣ��ѧʽ�� ��E��F��L��I���γɵļ����ӵİ뾶�ɴ�С��˳��Ϊ�������ӷ��ű�ʾ�� ��
��2����L��I��Ԫ�ؿɰ�ԭ�Ӹ�����1:1��ɻ�����X��������X�и�ԭ�Ӿ�����8���ӵ��ȶ��ṹ����X�ĵ���ʽΪ �����廯����E2D2Ͷ�뵽������E2L��ˮ��Һ�У�ֻ�۲쵽�г��������ģ�д���÷�Ӧ�����ӷ���ʽ ��
��3����10 L���ܱ������У�ͨ��2 mol LD2�����1 mol D2���壬һ���¶��·�Ӧ������LD3���壬����Ӧ�ﵽƽ��ʱ��D2��Ũ��Ϊ0.01 mol·L-1��ͬʱ�ų�Լ177 KJ����������ƽ��ʱLD2��ת����Ϊ ���÷�Ӧ���Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com