
H2��I2��һ���������ܷ�����Ӧ��H2(g) + I2(g) 2HI(g) ��H����a kJ��mol��1
2HI(g) ��H����a kJ��mol��1
��֪��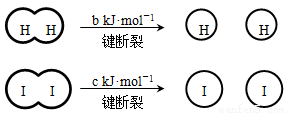 (a��b��c��������)
(a��b��c��������)
����˵������ȷ����( )
A����Ӧ��������������������������
B���Ͽ�1 mol H��H����1 mol I��I�������������ڶϿ�2 mol H��I����������
C���Ͽ�2 mol H��I����������ԼΪ(c+b+a) kJ
D�����ܱ������м���2 mol H2��2 mol I2����ַ�Ӧ��ų�������С��2a kJ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ʵ����
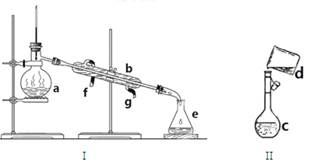
��1��д���������������ƣ�
a. b. c. e.
��2��������װ��I�������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ������� ������ˮ�� ����f��g����ͨ�롣
��3����������0.1mol/LNaOH��Һ450mL��װ��II��ijͬѧת����Һ��ʾ��ͼ��
��ͼ�еĴ����� ��
�ڸ��ݼ����֪����������ƽ�������NaOH������Ϊ g��
������ʱ������ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________��
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ |
B��������������������ƹ������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽⲢ��ȴ������ |
C�����ܽ������������Һ�ز�����ע��500mL������ƿ�� |
D��������ƿ�ǽ�����ҡ�� |
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2~3cm��
��4�����ʵ���Ũ��������������ƫ�ߡ�ƫ�͡���Ӱ�죩
������ֽ������������ ��
�ڶ���ʱ�����۾����ӿ̶��ߣ��������Ƶ���ҺŨ�Ƚ� ��
��δ��ȴ�����¾�ע������ƿ���� ��
����õ���Һת��ɾ����Լ�ƿʱ����������������Һ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��C��CO2 2CO������Ӧ���ȣ�����Ӧ����Ϊv1��N2��3H2
2CO������Ӧ���ȣ�����Ӧ����Ϊv1��N2��3H2 2
2 NH3������Ӧ���ȣ�����Ӧ����Ϊv2������������Ӧ�����¶�����ʱ��v1��v2�ı仯����ǣ� ��
NH3������Ӧ���ȣ�����Ӧ����Ϊv2������������Ӧ�����¶�����ʱ��v1��v2�ı仯����ǣ� ��
A��ͬʱ���� B��ͬʱ��С C��v1���ӣ�v2��С D��v1��С��v2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ��һ��10���¿���ѧ���������棩 ���ͣ�ѡ����
�����й�Na2CO3��NaHCO3˵���У���ȷ����( )
A�����߶�������ˮ���Ҷ��ܺ�ˮ��Ӧ��ǰ�ߵ��ܽ�ȴ��ں���
B�����߶�����������θ����༲����ǰ�ߵ�Ч�����ں���
C��������Na2CO3��NaHCO3�ֱ�������ʵ�����ϡ���ᷴӦ��ǰ�߲���CO2��
D���������Ũ�ȵ�AlCl3��Һ�ֱ��������Na2CO3��NaHCO3��Ӧ��ǰ�߲���CO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ��һ��10���¿���ѧ���������棩 ���ͣ�ѡ����
�����£����и���������ָ����Һ��һ���ܴ���������ǣ� ��
A��ʹ���ȱ��ɫ����Һ��Mg2+��K+��SO42����NO3��
B��ʹ��̪���ɫ����Һ��Na+��Cu2+��HCO3����NO3��
C��0.1 mol��L��1AgNO3��Һ��H+��K+��SO42����I��
D��0.1 mol��L��1NaAlO2��Һ��H+��Na+��Cl����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и߶��������Ļ�ѧ�Ծ��������棩 ���ͣ������
��ͼ��ʾװ�ã�
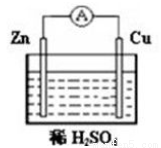
��1����ԭ���װ���и���Ϊ______________������______________��Ӧ������Ϊ___________������_____________��Ӧ��
��2��ԭ��ؽ�________��ת��Ϊ___________�ܡ�
��3�����������Ǵ�_________��___________�����������Ǵ�________��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и߶��������Ļ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��ȷ����
A������ˮ��Ӧ��Na+2H2O=Na++2OHһ+H2��
B��̼�����������ᷴӦ��CO32��+2H+=H2O+CO2��
C������ϡ���ᷴӦ��Fe+2H+=Fe3++H2��
D��������Һ������������Һ��Ӧ��CH3COOH+OHһ=CH3COOһ+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и߶�����������ѧ�Ծ��������棩 ���ͣ������
�ܶ�����̶������������
(1���� 25��ʱ����ʯī�缫���һ�����ı��� Na2CO3��Һ���������ĵ缫��ӦʽΪ___________�� �����缫��ӦʽΪ____________�� ��Һ pH___________(������С�䣩
(2����ⷨ��ȡ Na2FeO4��ͬʱ�ɻ��������Fe+2H2O+2OH- FeO42-+3H2��������ԭ����ͼ1��ʾ��װ��ͨ������缫���������Ϻ�ɫ�� FeO42-�����缫�����ݲ�����������������ҺŨ�ȹ��ߣ����缫����������ɫ���ʡ���֪��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ��
FeO42-+3H2��������ԭ����ͼ1��ʾ��װ��ͨ������缫���������Ϻ�ɫ�� FeO42-�����缫�����ݲ�����������������ҺŨ�ȹ��ߣ����缫����������ɫ���ʡ���֪��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ��
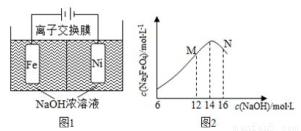
�� �����缫��Ӧʽ__________________�����һ��ʱ���c(OH-�����͵�������____________(������ҡ��������ҡ�����
�� �������У��뽫�������������弰ʱ�ų�����ԭ����________________��
��c(Na2FeO4�����ʼc(NaOH���ı仯��ͼ2������N��c(Na2FeO4���������ֵ��ԭ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶��������Ļ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ƻ��֭����ˮ����ɫ����ƻ��֭�ܻ�ԭ������Һ����˵���� ��
A��ƻ��ת��ʱ����ˮ��Ϊ���� B����ƻ����ֻ���е��Ƕ���������
C����ƻ����ֻ���е��۶��������� D��ƻ��ת��ʱ���Ǿۺϳɵ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com