
(1)[2013���������ۣ�11(2)]�绯ѧ����NO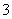 ��ԭ����ͼ��ʾ��
��ԭ����ͼ��ʾ��
�ٵ�Դ����Ϊ__________(�A����B��)��������ӦʽΪ
________________________________________________________________________��
������������ת����2 mol���ӣ���Ĥ������Һ�������仯��(��m������m��)Ϊ__________ g��
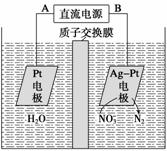
(2)[2013���������ۣ�23(2)��]����H2S������ȡ�����ķ����ж��֡����е绯ѧ����ͼ��ʾ����Ӧ���з�Ӧ��������������Һ������ʽ����Ŀ����________����Ӧ���з�����Ӧ�Ļ�ѧ����ʽΪ__________________����Ӧ�����Һ������أ�����ܷ�Ӧ�����ӷ���ʽΪ____________��
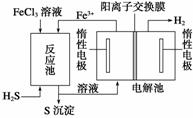
(3)[2013��ɽ�����ۣ�28(3)(4)]����ͼΪ��⾫������ʾ��ͼ��________(�a����b��)��Ϊ�������ʵĴ�������b������������ɫ��������������ɸ�����ĵ缫��ӦʽΪ________��
��Ϊ������������ĺڰ�(Ag2S)�����������������������ʳ ��ˮ�в������Ӵ���ʹAg2Sת��ΪAg��ʳ��ˮ��������________________��
��ˮ�в������Ӵ���ʹAg2Sת��ΪAg��ʳ��ˮ��������________________��
�𰸡�(1)��A��2NO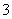 ��6H2O��10e��===N2����12OH��
��6H2O��10e��===N2����12OH��
��14.4
(2)����Ӧ��Ӵ������ʹ��Ӧ�����
H2S��2FeCl3===2FeCl2��S����2HCl
2Fe2����2H��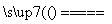 2Fe3����H2��
2Fe3����H2��
(3)��a��2H����NO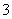 ��e��===NO2����H2O�������������Һ(��)
��e��===NO2����H2O�������������Һ(��)
������(1)��NO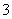 ����N2�����˻�ԭ��Ӧ��Ӧ�ڵ��ص�������������AΪԭ��ص�����������������ԭ��Ӧ���ʵ缫��ӦʽΪ2NO
����N2�����˻�ԭ��Ӧ��Ӧ�ڵ��ص�������������AΪԭ��ص�����������������ԭ��Ӧ���ʵ缫��ӦʽΪ2NO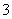 ��6H2O��10e��===N2����12OH��������������Ӧʽ��֪��ͨ��2 mol���ӣ���Һ���ٵ�����Ϊ5.6 g(N2)��ͬʱ��2 mol H��ͨ�����ӽ���Ĥ�����Ҳ࣬���Ҳ���Һ���� 3.6 g��
��6H2O��10e��===N2����12OH��������������Ӧʽ��֪��ͨ��2 mol���ӣ���Һ���ٵ�����Ϊ5.6 g(N2)��ͬʱ��2 mol H��ͨ�����ӽ���Ĥ�����Ҳ࣬���Ҳ���Һ���� 3.6 g��
���������ķ�ӦΪ4OH����4e��===O2����2H 2O��ÿͨ��2 mol���ӣ�����16 g O2��ͬʱ��2 mol H��ͨ�����ӽ���Ĥ�����Ҳ࣬ʹ�����Һ��������18 g����������Һ���ٵ�������Ϊ14.4 g��
2O��ÿͨ��2 mol���ӣ�����16 g O2��ͬʱ��2 mol H��ͨ�����ӽ���Ĥ�����Ҳ࣬ʹ�����Һ��������18 g����������Һ���ٵ�������Ϊ14.4 g��
(2)��������Һ������ʽ��Ŀ��������Ӧ��Ӵ������ʹ��Ӧ����֡���Ӧ���ڷ�����Ӧ�Ļ�ѧ����ʽΪH2S��2FeCl3===2FeCl2��S����2HCl��������ص�����ӦΪFeCl2��HCl�����ݵ���ͼʾ��������ɵ�ΪFe3�����Ҳ����ɵ�ΪH2����ɵ��ܷ�Ӧ�����ӷ���ʽ��2Fe2����2H��ͨ��,2Fe3����H2����
(3)�ٵ�⾫�����������������������������������ϳ��˸�����֮�⣬������������NO2���������ԭ����NO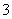 ��2H����e��===NO2����H2O��
��2H����e��===NO2����H2O��
��������������ʳ��ˮ����Ag2S��ԭ���ǣ�����ԭ���ԭ����������2Al��6e��===
2Al3������ ����3Ag2S��6e��===6Ag��3S2����2Al3����3S2����6H2O===2Al(OH)3����3H2S�����ܷ�Ӧ��2Al��3Ag2S��6H2O===2Al(OH)3����3H2S����6Ag��
����3Ag2S��6e��===6Ag��3S2����2Al3����3S2����6H2O===2Al(OH)3����3H2S�����ܷ�Ӧ��2Al��3Ag2S��6H2O===2Al(OH)3����3H2S����6Ag��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ҵ������еĸ��ֻ�ѧ����ͼ��ʾ�������Ҵ��ڸ��ַ�Ӧ�ж��Ѽ���˵������ȷ����(����)
 A���ͽ����Ʒ�Ӧʱ���ٶ���
A���ͽ����Ʒ�Ӧʱ���ٶ���
B����ͭ��������������O2��Ӧʱ���Ѣٺ͢�
C����ͭ��������������O2��Ӧʱ���Ѣٺ͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ö��Ե缫���һ��Ũ�ȵ�CuSO4��Һ��ͨ��һ��ʱ��������õ���Һ�м���0.1 mol Cu(OH)2��ǡ�ûָ������ǰ��Ũ�Ⱥ�pH��������˵����ȷ���� (����)
A��������������û����������
B����������ת�Ƶĵ��ӵ����ʵ���Ϊ0.4 mol
C��ԭCuSO4��Һ��Ũ��Ϊ0.1 mol��L��1
D���������������ռ������������Ϊ1.12 L(�����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�����ձ���ʢ�к�ˮ���������б���ʴ���ٶ��ɿ쵽����˳��Ϊ (����)


A���ڢ٢ۢܢݢ� B���ݢܢۢ٢ڢ�
C���ݢܢڢ٢ۢ� D���ݢۢڢܢ٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
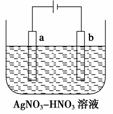
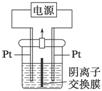 ���װ����ͼ��ʾ��������װ��KI��������Һ���м��������ӽ���Ĥ��������һ���ĵ�ѹ��ͨ�磬���������Һ����ɫ��һ��ʱ�����ɫ��dz��
���װ����ͼ��ʾ��������װ��KI��������Һ���м��������ӽ���Ĥ��������һ���ĵ�ѹ��ͨ�磬���������Һ����ɫ��һ��ʱ�����ɫ��dz��
��֪��3I2��6OH��===IO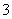 ��5I����3H2O
��5I����3H2O
����˵������ȷ���� (����)
A���Ҳ���ĵ缫��Ӧʽ��2H2O��2e��===H2����2OH��
B��������ʱ���Ҳ���Һ�к�IO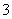
C�������ڷ�����Ӧ���ܻ�ѧ����ʽ��KI��3H2O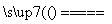 KIO3��3H2��
KIO3��3H2��
D������������ӽ���Ĥ���������ӽ���Ĥ�������ڷ������ܻ�ѧ��Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ֽ��Ȼ�����Һ�������ղ����ں��ò��缫���е�⣬�����йص缫������ж���ȷ���� (����)
A����������������
B����������������
C��������������������
D����������ֻ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ������������ȷ���� (����)
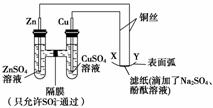
A��XΪ������������ԭ��Ӧ
B��YΪ����������������Ӧ
C��Y����ֽ�Ӵ�������������
D��X����ֽ�Ӵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������̬���Ե������ϣ���120 0Cʱ1 L�û������9 L������ϣ����ȼ�պ�ָ���ԭ״̬������������Ϊ10 L�����и��������в����ϴ��������� ( )
A��CH4��C2H4 B��C2H2��C3H8 C��C2H4��C3H4 D��C2H2��C3H6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���0.2mol/LAl2(SO4)3��Һ�У���μ���1.0mol/LNaOH��Һ��ʵ������ҺpH��NaOH��Һ����仯��������ͼ�������й�˵����ȷ����
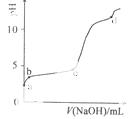 A��a��ʱ����Һ�����Ե�ԭ����Al3+ˮ�⣬���ӷ���ʽΪ��
A��a��ʱ����Һ�����Ե�ԭ����Al3+ˮ�⣬���ӷ���ʽΪ��
Al3++3OH- Al(OH)3
Al(OH)3
B��a��b�Σ���ҺpH����Al3+Ũ�Ȳ���
C��b��c�Σ������OH-��Ҫ��������Al(OH)3����
D��d��ʱ��Al(OH)3������ʼ�ܽ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com