
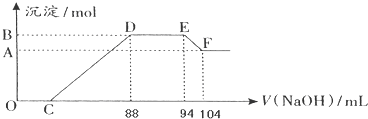
���� ͨ�����⣬��Ӧʼ��û���������ɣ����Եó������е������������ɣ����������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ������Ʋ�NԪ����+5�����-3�ۣ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬�ɵ���NH4+�����˷�Ӧ��
��1������NԪ���غ������������ʵ���
��2���ɸ���Al��OH��3 +OH-=AlO2-+2H2O���ó�Al��OH��3�����ʵ�����
��3��DE�η�Ӧ���ӷ���ʽΪ��NH4++OH-�TNH3•H2O��
��4��Al��OH��3�����ʵ���Ϊ0.05mol��������Ԫ���غ㣬n��Al��=0.05mol�����ݵ���ת���غ����n��Fe��=0.03mol���ݴ��������������������۵����ʵ���֮�ȣ�
��5���������Ϸ�����֪��n��Al��=0.05mol��n��NH4+��=0.03mol�����������ᷴӦʱ������Ļ�ԭ����ΪNH4NO3������������ԭ��Ӧ�غ���д��
��� �⣺ͨ�����⣬��Ӧʼ��û���������ɣ����Եó������е������������ɣ����������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ������Ʋ�NԪ����+5�����-3�ۣ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬�ɵ���NH4+�����˷�Ӧ��
��1��E��ʱ����Ϊ�����ơ�һˮ�ϰ�������NԪ���غ㣬��֪��n��HNO3��=n��NH3•H2O��+n��NaNO3��=0.94L��5mol/L+0.03mol=0.5mol��
�ʴ�Ϊ��0.5��
��2���ɸ���Al��OH��3 +OH-=AlO2-+2H2O���ó�Al��OH��3�����ʵ���Ϊ����104-94����10-3L��5mol/L=0.05 mol����A��B�IJ�ֵΪ0.05��
�ʴ�Ϊ��0.05��
��3��DE��Ϊ笠����������������ӷ�Ӧ����һˮ�ϰ�����Ӧ���ӷ���ʽΪ��NH4++OH-�TNH3•H2O��
�ʴ�Ϊ��NH4++OH-=NH3•H2O��
��4��Al��OH��3�����ʵ���Ϊ0.05mol��������Ԫ���غ㣬�ʻ�Ͻ�����n��Al��=0.05mol����ͼ��֪��DE�����ĵ��������Ƶ����Ϊ94mL-88mL=6mL���ʸýβμӷ�Ӧ����������Ϊ0.006L��5mol/L=0.03mol������NH4++OH-�TNH3•H2O ��֪��������Һ��n��NH4+��=0.03ml�����ݵ���ת���غ��У�3n��Fe��+3n��Al��=8n��NH4+������3n��Fe��+3��0.05mol=8��0.03mol����ã�n��Fe��=0.03mol����Ͻ�����n��Al��=0.05mol��n��Fe��=0.03mol����Ʒ�����ۺ����۵����ʵ���֮��Ϊ5��3��
�ʴ�Ϊ��5��3��
��5��������֪�����������ᷴӦʱ������Ļ�ԭ����ΪNH4NO3������������ԭ��Ӧ��ʧ�����غ�͵���غ㣬��Ӧ�����ӷ�ӦΪ��8Al+30H++3NO3-=8Al3++3NH4++9H2O��
�ʴ�Ϊ��8Al+30H++3NO3-=8Al3++3NH4++9H2O��
���� ���⿼������ļ��㣬��Ŀ�ѶȽϴ���ע�����ͼ���жϸ��η�Ӧ���������ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬ע��������غ�ĽǶȽ�Ϸ�Ӧ�Ĺ�ϵʽ���㣬����������ѧ���Ļ�ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | +1 | B�� | +2 | C�� | +3 | D�� | +4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʳ��� | B�� | ʳ����� | C�� | ��ѩ���� | D�� | ʯ���ѽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��Cl-��=c��NH3•H2O��+c��NH4+�� | |
| B�� | c��Cl-����c��NH4+����c��Na+����c��NH3•H2O����c��OH-����c��H+�� | |
| C�� | c��Cl-��+c��OH-��=c��Na+��+c��NH4+��+c��H+�� | |
| D�� | c��NH3•H2O��+c��OH-��=c��Na+��+c��NH4+��+c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʹpH��ֽ�ʺ�ɫ����Һ��Na+��NH4+��I-��NO3- | |
| B�� | ������pH=12����Һ��Na+��K+��SiO32-��NO3- | |
| C�� | c��Fe3+��=0.1mol•L-1����Һ��H+��Al3+��I-��SCN- | |
| D�� | ������������H2����Һ��K+��Mg2+��SO42-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 $?_{��}^{Ũ����}$
$?_{��}^{Ũ����}$ +H2O���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ��ȡ����Ӧ
+H2O���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ��ȡ����Ӧ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| F | Cl | Br | I | |
| ��һ�����ܣ���kJ•mol-1���� | 1681 | 1251 | 1140 | 1008 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ϊ222 | |
| B�� | ������Ϊ86 | |
| C�� | ������Ϊ308 | |
| D�� | ��${\;}_{86}^{219}$Rn��${\;}_{86}^{220}$Rn��Ϊͬλ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com