
���ྻ�Ľ���Ƭ�ס��ҡ��������ֱ�����ڽ���ij������Һ����ֽ�ϲ�ѹ��������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹ��ָ����ƶ�����͵�ѹ���Ķ������±�������֪�������缫�Ľ�����������������Խ��ѹ���Ķ���Խ��
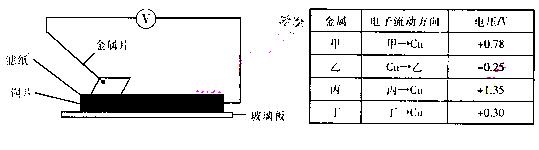
���ݼ�¼�����жϣ����н�������ȷ����
A�����ס����γɵĺϽ�¶���ڿ����У����ȱ���ʴ
B���������ܴ�����ͭ��Һ���û���ͭ
C�������ֽ����б��Ļ�ԭ������
D���ס������γ�ԭ���ʱ����Ϊ����
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ҹ���һ��������ߡ����������У������ڻ������ǣ�������
A�������ŷ���ˮ B���ƹ�ʹ������ú
C����������β���ŷ� D���ƹ�ʹ������ϴ�Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���±���S2X2���ǹ㷺������ҵ������S2C12���ӽṹ��H2O2���ơ���ˮ��ˮ�⣬��������ʹƷ����ɫ�����壬��ѧ����ʽΪ��2S2C12+2H2O��SO2��+3S��+4HCl������˵���д������
A���ȶ���S2C12��S2Br2
B����Ӧ�У�����1molSO2��ת�Ƶ���Ϊ3mol
C��������ͬʱ���м��Լ��ͷǼ��Լ��Ҹ�ԭ�Ӷ�����8�����ȶ��ṹ
D����ΪS��Cl���ܱ�S��Br���ܴ�S2C12�е��S2Br2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A��ǿ����ʵ�ˮ��Һ�в��������ʷ��ӣ�������ʵ�ˮ��Һ�д������ʷ��Ӻ�����
B��ǿ����ʵ�ˮ��Һ������ǿ��������ʵ�ˮ��Һ
C��ǿ����ʶ������ӻ����������ʶ��ǹ��ۻ�����
D��ǿ�����������ˮ���������������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С����Ƴ����ù�ҵ����(l0%)���ѽ�ij����������ͭп��ķ�����ʵ�ַ����ۺ����ã�����ͼ��ʾ��
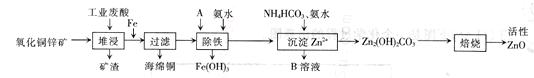
��֪�������ӿ�ʼ��������ȫ����ʱ��pH���±���ʾ��

��ش��������⣺
(1)����ͭп���к���������CuS��ZnS����H2SO4��������ZnS�����ܽ��CuS
���ܣ�����ͬ�¶��£�Ksp(CuS)______Ksp(ZnS)��ѡ�>����<����=������
(2)����A��ʹ�����������е� ��
A.KMnO4 B.O2 C.H2O2 D.Cl2
(3)���������м��백ˮ��Ŀ���ǵ�����Һ��pH��pHӦ������________��Χ֮�䡣
(4)����B��ֱ���������ʣ���B�Ļ�ѧʽ��________��
(5)������õ���Fe(OH)3����KClO��Һ�ڼ��Ի����½��������õ�һ�ָ�Ч�Ķ��ˮ����������K2FeO4��д���÷�Ӧ�����ӷ���ʽ__________��
�塢ѡ���⣨��26��27��ѡ��һ�⽫��ȷ��д�ڴ���ϣ���15�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и����Ȼ�ѧ����ʽ�У���ѧ��Ӧ�Ħ�Hǰ�ߴ��ں��ߵ��� (����)
��C(s)��O2(g)===CO2(g)����H1
C(s)�� O2(g)===CO(g)����H2
O2(g)===CO(g)����H2
��S(s)��O2(g)===SO2(g)����H3
S(g)��O2(g)===SO2(g)����H4
��H2(g)�� O2(g)===H2O(l)����H5
O2(g)===H2O(l)����H5
2H2(g)��O2(g)===2H2O(l)����H6
��CaCO3(s)===CaO(s)��CO2(g)����H7
CaCO(s)��H2O(l)===Ca(OH)2(s)����H8
A���� B����
C���ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)�����ܸı䷴Ӧ���ʱ� (����)
(2012·���գ�4B)
(2)�����ܽ��ͷ�Ӧ�Ļ�� (����)
(2012·���գ�4C)
(3)����̫�����ڴ��������·ֽ�ˮ�����ǰѹ���ת��Ϊ��ѧ�ܵ���ɫ��ѧ����(����)
(2012·�㽭���ۣ�7A)
(4)��ѧ��Ӧ�����������ɣ�����ѭ�����غ㶨�ɺ������غ㶨�� (����)
(2011·�㽭���ۣ�7A)
(5)ʵ���û�����(l)������ϩ(l)�ͱ�(l)�ı�ȼ���ȷֱ�Ϊ��3 916 kJ·mol��1��
��3 747 kJ·mol��1�ͣ�3 265 kJ·mol��1������֤���ڱ������в����ڶ�����̼̼˫��(����)
(2011·�㽭���ۣ�12C)
(6)��֪��Fe2O3(s)��3C(ʯī)===2Fe(s)��3CO(g)
��H��489.0 kJ·mol��1
CO(g)�� O2(g)===CO2(g)
O2(g)===CO2(g)
��H����283.0 kJ·mol��1
C(ʯī)��O2(g)===CO2(g)
��H����393.5 kJ·mol��1
��4Fe(s)��3O2(g)===2Fe2O3(s)
��H����1 641.0 kJ·mol��1 (����)
(2011·�㽭���ۣ�12D)
(7)�������ܡ�̫���ܵ�����Դ���ƹ�״����ͣ�ʹ������ϴ�Ӽ�����ֱ�ӽ���̼�ŷ�
(����)
(2010·�㽭���ۣ�7B)
(8)500 �桢300 MPa�£���0.5 mol N2��1.5 mol H2�����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪN2(g)��3H2(g)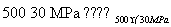 2NH3(g)����H��
2NH3(g)����H��
��38.6kJ·mol��1 (����)
(2010·�㽭���ۣ�12B)
(9)ʹ��̫������ˮ�������������á��������Ҵ����漰���������ܵ����� (����)
(2009·�㽭���ۣ�7C)
(10)ʯ�͡�ú����Ȼ������ȼ����ֲ���Ͷ����ڻ�ʯȼ�� (����)
(2009·�㽭���ۣ�7D)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com