
(1)���Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ�___________________________________________________________________��
Ȼ���������Թܣ�ʹ֮��Ͼ��ȡ�
(2)װ����ͨ�����ĵ���Ҫ���ڱ���Na2CO3��Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹNa2CO3��Һ��������ɵ����IJ����ϵ�ԭ����_____________________________��
(3)Ũ����������ǣ���__________________����__________________��
(4)����Na2CO3��Һ��������____________________________________________________��
(5)ʵ�����ɵ��������������ܶȱ�ˮ___________��(���С��)����___________��ζ��
(6)��ʵ�����¶ȹ��ߣ�ʹ��Ӧ�¶ȴﵽ
������(1)ŨH2SO4�����Ҵ��������ų��������ȣ������Ҵ���������ܶȶ�С�����ᣬ���Բ��ܽ��Ҵ���������뵽Ũ�����С�
(2)�ƾ��ƵĻ����ܿ���������Ӱ�죬��ǿ�����������Թ���Һ�����Ȳ����ȣ������ܲ��ڱ���Na2CO3��Һ��Һ�����£��ͻ�����Һ��ĵ�����
(4)ͬ��������һ������������Ҵ�������������ҺNa2CO3�����ܽ��Ҵ��������ᷴӦ��ͬʱ���ܼ�������ˮ�е��ܽ⣬��������ˮ�ֲ㡣
(5)�����ܶȱ�ˮС�����������й���ζ��
(6)�¶ȴﵽ
�𰸣�(1)��Ũ�������������Ҵ�������Ļ��Һ��
(2)���Ȳ�����
(3)���� ��ˮ��
(4)�����Ҵ�����Ӧ���ᣬ���������ܽ⣬�����ڷֲ�
(5)С ����
(6)��ϩ


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
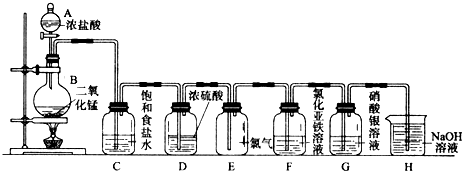
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
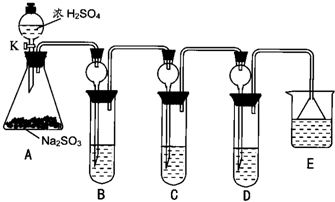
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
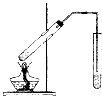 ʵ��������ͼ��ʾװ����ȡ����������
ʵ��������ͼ��ʾװ����ȡ����������| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |



| ���� |
| ���� |
| Cu��Ag |
| �� |
| Cu��Ag |
| �� |

| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com