
ČēĶ¼±ķŹ¾ĶłÄ³Na2CO3ČÜŅŗÖŠµĪČėŃĪĖį¼°²śÉśCO2µÄ¹ż³Ģ£ŗ
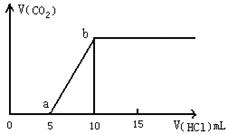
£Ø1£©Š“³öaµćŅŌĒ°·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
£Ø2£©Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚaµćŹ±£¬ŌŁĻņČÜŅŗÖŠ¼ÓČėAl3+ČÜŅŗ£¬Ōņ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
£»
£Ø3£©Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚbµćŹ±£¬ÓƶčŠŌµē¼«µē½āøĆČÜŅŗµÄ×Ü·“Ó¦·½³ĢĪŖ ___
£»
µ±Į½¼«Éś³ÉĘųĢåµÄĢå»ż¾łŹĒ1.12L£Ø±ź×¼×“æöĻĀ£©Ź±£¬¼ÓČė mol £ØĢīĆū³Ę£©Ē”ŗĆ½«ŌČÜŅŗø“Ō£»
£Ø4£©ČōijČÜŅŗÖŠŗ¬1 mol NaHCO3£¬µĪČėŅ»¶ØĮæµÄĻ”NaOH£¬Ē”ŗĆŹ¹ČÜŅŗÖŠNa+ ŗĶHCO3- µÄĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ1£¬ŌņµĪČėµÄNaOHµÄĪļÖŹµÄĮæĪŖ mol”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ¼ŖĮÖŹ”³¤“ŗŹŠµŚŹ®Ņ»ÖŠŃ§2011½ģøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗ038
ČēĶ¼±ķŹ¾ĶłÄ³
Na2CO3ČÜŅŗÖŠµĪČėŃĪĖį¼°²śÉśCO2µÄ¹ż³Ģ£ŗ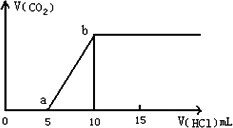
(1)Š“³öaµćŅŌĒ°·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½________£»
(2)Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚaµćŹ±£¬ŌŁĻņČÜŅŗÖŠ¼ÓČėAl3+ČÜŅŗ£¬Ōņ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________£»
(3)Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚbµćŹ±£¬ÓƶčŠŌµē¼«µē½āøĆČÜŅŗµÄ×Ü·“Ó¦·½³ĢĪŖ________£»
µ±Į½¼«Éś³ÉĘųĢåµÄĢå»ż¾łŹĒ1.12 L(±ź×¼×“æöĻĀ)Ź±£¬¼ÓČė________mol________(ĢīĆū³Ę)Ē”ŗĆ½«ŌČÜŅŗø“Ō£»
(4)ČōijČÜŅŗÖŠŗ¬1 mol””NaHCO3£¬µĪČėŅ»¶ØĮæµÄĻ”NaOH£¬Ē”ŗĆŹ¹ČÜŅŗÖŠNa+ŗĶHCO![]() µÄĪļÖŹµÄĮæÖ®±ČĪŖ2”Ć1£¬ŌņµĪČėµÄNaOHµÄĪļÖŹµÄĮæĪŖ________mol£®
µÄĪļÖŹµÄĮæÖ®±ČĪŖ2”Ć1£¬ŌņµĪČėµÄNaOHµÄĪļÖŹµÄĮæĪŖ________mol£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼±ķŹ¾ĶłÄ³Na2CO3ČÜŅŗÖŠµĪČėŃĪĖį¼°²śÉśCO2µÄ¹ż³Ģ£ŗ
£Ø1£©Š“³öaµćŅŌĒ°·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
£Ø2£©Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚaµćŹ±£¬ŌŁĻņČÜŅŗÖŠ¼ÓČėAl3+ČÜŅŗ£¬Ōņ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
£»
£Ø3£©Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚbµćŹ±£¬ÓƶčŠŌµē¼«µē½āøĆČÜŅŗµÄ×Ü·“Ó¦·½³ĢĪŖ ___
£»
µ±Į½¼«Éś³ÉĘųĢåµÄĢå»ż¾łŹĒ1.12L£Ø±ź×¼×“æöĻĀ£©Ź±£¬¼ÓČė mol £ØĢīĆū³Ę£©Ē”ŗĆ½«ŌČÜŅŗø“Ō£»
£Ø4£©ČōijČÜŅŗÖŠŗ¬1 mol NaHCO3£¬µĪČėŅ»¶ØĮæµÄĻ”NaOH£¬Ē”ŗĆŹ¹ČÜŅŗÖŠNa+ ŗĶHCO3- µÄĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ1£¬ŌņµĪČėµÄNaOHµÄĪļÖŹµÄĮæĪŖ mol”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ¼ŖĮÖŹ”³¤“ŗŹŠŹ®Ņ»øßÖŠ2010-2011ѧğ¶ČøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼±ķŹ¾ĶłÄ³Na2CO3ČÜŅŗÖŠµĪČėŃĪĖį¼°²śÉśCO2µÄ¹ż³Ģ£ŗ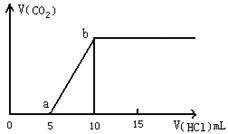
£Ø1£©Š“³öaµćŅŌĒ°·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
£Ø2£©Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚaµćŹ±£¬ŌŁĻņČÜŅŗÖŠ¼ÓČėAl3+ČÜŅŗ£¬Ōņ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
£»
£Ø3£©Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚbµćŹ±£¬ÓƶčŠŌµē¼«µē½āøĆČÜŅŗµÄ×Ü·“Ó¦·½³ĢĪŖ ___
£»
µ±Į½¼«Éś³ÉĘųĢåµÄĢå»ż¾łŹĒ1.12L£Ø±ź×¼×“æöĻĀ£©Ź±£¬¼ÓČė mol £ØĢīĆū³Ę£©Ē”ŗĆ½«ŌČÜŅŗø“Ō£»
£Ø4£©ČōijČÜŅŗÖŠŗ¬1 mol NaHCO3£¬µĪČėŅ»¶ØĮæµÄĻ”NaOH£¬Ē”ŗĆŹ¹ČÜŅŗÖŠNa+ ŗĶHCO3-µÄĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ1£¬ŌņµĪČėµÄNaOHµÄĪļÖŹµÄĮæĪŖ mol”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ¼ŖĮÖŹ”³¤“ŗŹŠ2011½ģ¶ČøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼±ķŹ¾ĶłÄ³Na2CO3ČÜŅŗÖŠµĪČėŃĪĖį¼°²śÉśCO2µÄ¹ż³Ģ£ŗ
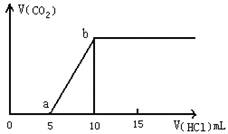
£Ø1£©Š“³öaµćŅŌĒ°·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
£Ø2£©Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚaµćŹ±£¬ŌŁĻņČÜŅŗÖŠ¼ÓČėAl3+ČÜŅŗ£¬Ōņ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
£»
£Ø3£©Čō¼ÓĖįÖĮĶ¼Ļń“¦ÓŚbµćŹ±£¬ÓƶčŠŌµē¼«µē½āøĆČÜŅŗµÄ×Ü·“Ó¦·½³ĢĪŖ ___
£»
µ±Į½¼«Éś³ÉĘųĢåµÄĢå»ż¾łŹĒ1.12L£Ø±ź×¼×“æöĻĀ£©Ź±£¬¼ÓČė mol £ØĢīĆū³Ę£©Ē”ŗĆ½«ŌČÜŅŗø“Ō£»
£Ø4£©ČōijČÜŅŗÖŠŗ¬1 mol NaHCO3£¬µĪČėŅ»¶ØĮæµÄĻ”NaOH£¬Ē”ŗĆŹ¹ČÜŅŗÖŠNa+ ŗĶHCO3- µÄĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ1£¬ŌņµĪČėµÄNaOHµÄĪļÖŹµÄĮæĪŖ mol”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com