
ŅŃÖŖ£ŗCu(OH)2ŹĒ¶žŌŖČõ¼ī£»ŃĒĮ×Ėį£ØH3PO3£©ŹĒ¶žŌŖČõĖį£¬ÓėNaOHČÜŅŗ·“Ó¦£¬Éś³ÉNa2HPO3”£
£Ø1£©ŌŚĶŃĪČÜŅŗÖŠCu2£«·¢ÉśĖ®½ā·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ____£»£ØŅŃÖŖ£ŗ25”ꏱ£¬Ksp[Cu(OH)2]£½2.0”Į10£20mol3/L3£©
£Ø2£©øł¾ŻH3PO3µÄŠŌÖŹæÉĶĘ²āNa2HPO3Ļ”ČÜŅŗµÄpH______7£ØĢī”°£¾”±”°£¼”±»ņ”°£½”±£©”£³£ĪĀĻĀ£¬Ļņ10mL0.01mol/L H3PO3ČÜŅŗÖŠµĪ¼Ó10ml0.02mol/LNaOHČÜŅŗŗó£¬ČÜŅŗÖŠø÷ÖÖĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ_________£»
£Ø3£©µē½āNa2HPO3ČÜŅŗæɵƵ½ŃĒĮ×Ėį£¬×°ÖĆČēĶ¼£ØĖµĆ÷£ŗŃōĤֻŌŹŠķŃōĄė×ÓĶعż£¬ŅõĤֻŌŹŠķŅõĄė×ÓĶعż£©
¢ŁŃō¼«µÄµē¼«·“Ó¦Ź½ĪŖ____________________”£
¢Ś²śĘ·ŹŅÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________”£
£Ø13·Ö£©£Ø1£©Cu2+£«2H2O Cu(OH)2£«2H+£Ø2·Ö£©£»5”Į10£9£Ø2·Ö£©
Cu(OH)2£«2H+£Ø2·Ö£©£»5”Į10£9£Ø2·Ö£©
£Ø2£©£¾£Ø2·Ö£©£»c(Na+)£¾c(HPO32£)£¾c(OH£)£¾c(H2PO3£)£¾c(H+)£Ø2·Ö£©
£Ø3£©¢Ł4OHØDØD4e££½2H2O£«O2”ü£Ø2·Ö£©
¢ŚHPO32££«2H£«£½H3PO3£Ø2·Ö£©»ņHPO32££«H£«£½H2PO3£”¢H2PO3££«H£«£½H3PO3£Øø÷1·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ĒāŃõ»ÆĶŹĒČõ¼ī£¬ĖłŅŌĶĄė×ÓæÉŅŌĖ®½ā£¬ČÜŅŗĻŌĖįŠŌ£¬ĘäĖ®½ā·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖCu2+£«2H2O Cu(OH)2£«2H+”£»ÆŃ§Ę½ŗā³£ŹżŹĒŌŚŅ»¶ØĢõ¼žĻĀ£¬µ±æÉÄę·“Ó¦“ļµ½Ę½ŗāדĢ¬Ź±£¬Éś³ÉĪļÅØ¶ČµÄĆŻÖ®»żŗĶ·“Ó¦ĪļÅØ¶ČµÄĆŻÖ®»żµÄ±ČÖµ£¬ĖłŅŌøĆ·“Ó¦µÄĘ½ŗā³£ŹżK£½
Cu(OH)2£«2H+”£»ÆŃ§Ę½ŗā³£ŹżŹĒŌŚŅ»¶ØĢõ¼žĻĀ£¬µ±æÉÄę·“Ó¦“ļµ½Ę½ŗāדĢ¬Ź±£¬Éś³ÉĪļÅØ¶ČµÄĆŻÖ®»żŗĶ·“Ó¦ĪļÅØ¶ČµÄĆŻÖ®»żµÄ±ČÖµ£¬ĖłŅŌøĆ·“Ó¦µÄĘ½ŗā³£ŹżK£½ £½
£½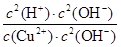 =
=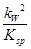 £½
£½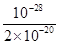 £½5”Į10£9”£
£½5”Į10£9”£
£Ø2£©H3PO3ŹĒČõĖį£¬Na2HPO3ŹĒĒæ¼īČõĖįŃĪ£¬ĖłŅŌHPO32£Ė®½ā£¬ĘäĖ®ČÜŅŗ³Ź¼īŠŌ£¬¼“pH£¾7£»Ļņ10mL0.01mol/LH3PO3ČÜŅŗÖŠµĪ¼Ó10ml 0.02mol/LNaOHČÜŅŗŗ󣬶žÕßĒ”ŗĆ·“Ӧɜ³ÉNa2HPO3£¬ČÜŅŗĖ®½āĻŌ¼īŠŌ£¬ĖłŅŌČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”ĪŖc(Na+)£¾c(HPO32£)£¾c(OH£)£¾c(H2PO3£)£¾c(H+)”£
£Ø3£©¢Łµē½ā³ŲŃō¼«Ź§Č„µē×Ó£¬·¢ÉśŃõ»Æ·“Ó¦£¬Ņõ¼«µĆµ½µē×Ó·¢Éś»¹Ō·“Ó¦”£ĖłŅŌøł¾Ż×°ÖĆĶ¼æÉÖŖ£¬Ńō¼«ÉĻĒāŃõøłĄė×ÓŹ§µē×Ó·¢ÉśŃõ»Æ·“Ó¦£¬µē¼«·“Ó¦Ź½ĪŖ4OHØDØD4e££½2H2O£«O2”ü”£
¢ŚÓÉÓŚŃōĤֻŌŹŠķŃōĄė×ÓĶعż£¬ŅõĤֻŌŹŠķŅõĄė×ÓĶعż£¬ĖłŅŌ²śĘ·ŹŅÖŠHPO32£ŗĶĒāĄė×Ó½įŗĻÉś³ÉŃĒĮ×Ėį£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖHPO32££«2H£«£½H3PO3£¬»ņHPO32££«H£«£½H2PO3£”¢H2PO3££«H£«£½H3PO3”£
æ¼µć£ŗæ¼²éĖ®½ā·½³ĢŹ½”¢Ę½ŗā³£Źż¼ĘĖć£»Ėį¼īÖŠŗĶČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”±Č½Ļ£»µē½āŌĄķµÄÓ¦ÓĆŅŌ¼°µē¼«·“Ó¦Ź½µÄŹéŠ“µČ


 ĄųŌÅŹéŅµŹī¼ŁĻĪ½ÓÄž²Ø³ö°ęÉēĻµĮŠ“š°ø
ĄųŌÅŹéŅµŹī¼ŁĻĪ½ÓÄž²Ø³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(1)Ė®µÄµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£¬ČōAµć±ķŹ¾25”ꏱĖ®µÄµēĄė“ļĘ½ŗāŹ±µÄĄė×ÓÅØ¶Č£¬Bµć±ķŹ¾100”ꏱĖ®µÄµēĄė“ļĘ½ŗāŹ±µÄĄė×ÓÅØ¶Č”£Ōņ100”ꏱ1 mol”¤L£1µÄNaOHČÜŅŗÖŠ£¬ÓÉĖ®µēĄė³öµÄc(H£«)£½___________mol”¤L£1”£25 ”ꏱ£¬ĻņĖ®µÄµēĄėĘ½ŗāĢåĻµÖŠ¼ÓČėÉŁĮæNH4Cl¹ĢĢ壬¶ŌĖ®µÄµēĄėĘ½ŗāµÄÓ°ĻģŹĒ________(Ģī”°“Ł½ų”±”¢”°ŅÖÖĘ”±»ņ”°²»Ó°Ļģ”±)”£
(2)µēĄėĘ½ŗā³£ŹżŹĒŗāĮæČõµē½āÖŹµēĄė³Ģ¶ČĒæČõµÄĮ攣ŅŃÖŖČē±ķŹż¾Ż”£
| »ÆѧŹ½ | µēĄėĘ½ŗā³£Źż(25”ę) |
| HCN | K£½4.9”Į10£10 |
| CH3COOH | K£½1.8”Į10£5 |
| H2CO3 | K1£½4.3”Į10£7”¢K2£½5.6”Į10£11 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĮņĖįŹĒĒæĖį£¬ÖŠŃ§½×¶Ī½«ĮņĖįŌŚĖ®ČÜŅŗÖŠæ“×÷ĶźČ«µēĄė”£µ«ŹĀŹµŹĒ£¬ĮņĖįŌŚĖ®ÖŠµÄµŚŅ»²½µēĄėŹĒĶźČ«µÄ£¬µŚ¶ž²½µēĄė²¢²»ĶźČ«£¬ĘäµēĄėĒéæöĪŖ:H2SO4=H++HSO4-£¬HSO4- H+ + S042-”£
H+ + S042-ӣ
Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā:
£Ø1£©Na2SO4ČÜŅŗ³Ź_(Ģī”°ČõĖįŠŌ”±”¢”°ÖŠŠŌ”±»ņ”°Čõ¼īŠŌ”±)£¬ĘäĄķÓÉŹĒ_
(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
£Ø2£©H2SO4ČÜŅŗÓėBaC12ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_ ”£
£Ø3£©ŌŚ0£®l0mol”¤L-1µÄNa2SO4ČÜŅŗÖŠ£¬ĻĀĮŠĄė×ÓÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ_ (ĢīŠ“±ąŗÅ)”£
| A£®c(Na+)=c(SO42-)+c£ØHSO4Ņ»)+c(H2SO4) |
| B£®c(OH-)="c(" HSO4-)+c(H+) |
| C£®c( Na+)+c(H+)=c(OH-)+c(HSO4-)+2c(SO42-) |
| D£®c( Na+)=2c(SO42-)+2c(HSO4-) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
[14·Ö]ŅŃÖŖ£ŗI2£«2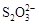

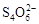 £«2IØD”£Ļą¹ŲĪļÖŹµÄČܶȻż³£Źż¼ūĻĀ±ķ£ŗ
£«2IØD”£Ļą¹ŲĪļÖŹµÄČܶȻż³£Źż¼ūĻĀ±ķ£ŗ
| ĪļÖŹ | Cu(OH)2 | Fe(OH)3 | CuCl | CuI |
| Ksp | 2.2”Į10£20 | 2.6”Į10£39 | 1.7”Į10£7 | 1.3”Į10£12 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ŌĖÓĆ»Æѧ·“Ó¦ŌĄķŃŠ¾æĢ¼”¢µŖ”¢Įņ”¢Āȵȵ„ÖŹ¼°Ęä»ÆŗĻĪļµÄ·“Ó¦£¬¶ŌÉś²ś”¢Éś»ī”¢»·¾³±£»¤µČĮģÓņÓŠ×ÅÖŲŅŖµÄŅāŅ唣
£Ø1£©ĻĀĮŠ“ėŹ©ÖŠ£¬²»ĄūÓŚ»·¾³±£»¤µÄÓŠ £ØĢī±ąŗÅ£©”£
a.“óĮææŖ²ÉŹ¹ÓĆ»ÆŹÆČ¼ĮĻ
b.Ź¹ÓĆĪŽ·ś±łĻ䔢æÕµ÷
c.¶ą²½ŠŠ¶ą³Ė¹«½»³µ£¬ÉŁÓĆ×سµŗĶĖ½¼Ņ³µ
d.½«¹¤Ņµ”°·ĻĘų”±”¢”°·ĻŅŗ”±”¢”°·ĻŌü”±Ö±½ÓÅÅ·Å
£Ø2£©¹¤ŅµÉĻµÄ”°ĶŃĢ¼”±ÖøµÄŹĒ“Ó”°ĶŃĻõ”±”¢”°ĶŃĮņ”±ŗóµÄŃĢĘųÓĆ¼īŅŗĪüŹÕ²¢µĆµ½ÅØĖõµÄ¶žŃõ»ÆĢ¼”£ĄūÓƶžŃõ»ÆĢ¼ŗĻ³É¼×“¼ŹĒĢ¼¼õÅŵĊĀ·½Ļņ”£
¢ŁŠ“³öĪüŹÕ¶žŃõ»ÆĢ¼µÄĄė×Ó·½³ĢŹ½ ”£
¢Ś³£ĪĀĻĀ£¬0.1mol/LNaHCO3ČÜŅŗµÄpH£¾8£¬ŌņČÜŅŗÖŠc(H2CO3) _______c(CO32£) £ØĢī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©”£
¢ŪŗĻ³ÉµÄ¼×“¼æÉŅŌ×öĪŖŠĀŠĶČ¼ĮĻµē³ŲµÄŌĮĻ£¬Čōµē½āŅŗŹĒ¼īŠŌµÄ£¬ŌņĘäøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø3£©¶žŃõ»ÆĀČ£ØClO2£©£¬ĪŖŅ»ÖÖ»ĘĀĢÉ«ĘųĢ壬ŹĒ¹«ČĻµÄøߊ§”¢¹ćĘ×°²Č«µÄɱ¾śĻū¶¾¼Į”£¹¤ŅµÉĻÖʱøClO2µÄ·“Ó¦ŌĄķĪŖ£ŗ4HC1(ÅØ)+2NaClO3£½2ClO2”ü+Cl2”ü+2H2O+2NaCl”£ÉĻŹö·“Ó¦ÖŠ£¬²śÉś1 mol ClO2£¬Ōņ±»Ńõ»ÆµÄHC1ĪŖ ”£
£Ø4£©SO2ČÜÓŚĖ®æÉŅŌµĆµ½¶žŌŖČõĖįH2SO3£ØŃĒĮņĖį£©”£
¢Ł25”ꏱ£¬½«NaOHÉīŅŗÓėŃĒĮņĖį»ģŗĻÖĮĒ”ŗĆÖŠŗĶ£¬Ōņ»ģŗĻŅŗÖŠø÷ÖÖĄė×ÓÅØ¶ČµÄ“óŠ”¹ŲĻµĪŖ ”£
¢Ś25”ꏱ£¬µ±NaOHČÜŅŗÓėH2SO3µČĪļÖŹµÄĮæ»ģŗĻŹ±£¬·¢ĻÖ»ģŗĻŅŗpH£¼7£¬ĒėÄć¼ņŅŖ½āŹĶĘäŌŅņ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ĮŠŹ½²¢¼ĘĖćĻĀĮŠø÷Š”Ģā£ŗ
£Ø1£©Ä³ĪĀ¶ČŹ±£¬²āµĆ0.01 mol/LµÄNaOHČÜŅŗpHĪŖ11£¬ĒóøĆĪĀ¶ČĻĀĖ®µÄĄė×Ó»ż³£ŹżKw
£Ø2£©ŌŚ“ĖĪĀ¶ČĻĀ£¬½«pH£½aµÄNaOHČÜŅŗVa LÓėpH£½bµÄĮņĖįVb L»ģŗĻ”£
¢ŁČōĖłµĆ»ģŗĻČÜŅŗĪŖÖŠŠŌ£¬ĒŅa£½12£¬b£½2£¬ĒóVa”ĆVb”£
¢ŚČōĖłµĆ»ģŗĻČÜŅŗµÄpH£½10£¬ĒŅa£½12£¬b£½2£¬ĒóVa”ĆVb”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
»ÆѧÄÜÓėµēÄÜÖ®¼äµÄĻą»„×Ŗ»ÆÓėČĖµÄÉś»īŹµ¼ŹĆÜĒŠĻą¹Ų£¬ŌŚÉś²ś”¢Éś»īÖŠÓŠÖŲŅŖµÄÓ¦ÓĆ£¬Ķ¬Ź±Ņ²ŹĒѧɜŠĪ³É»ÆѧѧæĘĖŲŃųµÄÖŲŅŖ×é³É²æ·Ö”£
£Ø1£©ČŪȌדĢ¬ĻĀ£¬Äʵĵ„ÖŹŗĶĀČ»ÆŃĒĢśÄÜ×é³Éæɳäµēµē³Ų(ČēĶ¼1)£¬·“Ó¦ŌĄķĪŖ£ŗ2Na£«FeCl2  Fe£«2NaCl,øƵē³Ų·ÅµēŹ±£¬Õż¼«·“Ó¦Ź½ĪŖ ________________ _____£ŗ
Fe£«2NaCl,øƵē³Ų·ÅµēŹ±£¬Õż¼«·“Ó¦Ź½ĪŖ ________________ _____£ŗ
³äµēŹ±£¬__________(Š“ĪļÖŹĆū³Ę)µē¼«½ÓµēŌ“µÄøŗ¼«£»
øƵē³ŲµÄµē½āÖŹĪŖ________ _”£
£Ø2£©Ä³Ķ¬Ń§ÓĆĶʬ”¢ŹÆÄ«×÷µē¼«µē½āŅ»¶ØÅØ¶ČµÄĮņĖįĶČÜŅŗ(ČēĶ¼2)£¬Ņ»¶ĪŹ±¼äĶ£Ö¹ĶصēČ”³öµē¼«”£ČōŌŚµē½āŗóµÄČÜŅŗÖŠ¼ÓČė0.98gĒāŃõ»ÆĶ·ŪÄ©Ē”ŗĆĶźČ«Čܽā£¬¾²ā¶ØĖłµĆČÜŅŗÓėµē½āĒ°ĶźČ«ĻąĶ¬”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁYµē¼«²ÄĮĻŹĒ £¬·¢Éś (Ģī”°Ńõ»Æ»ņ»¹Ō”±)·“Ó¦”£
¢Śµē½ā¹ż³ĢÖŠXµē¼«ÉĻ·¢ÉśµÄµē¼«·“·½Ó¦Ź½ŹĒ£ŗ
¢ŪČēŌŚµē½āŗóµÄČÜŅŗÖŠ¼ÓČė×ćĮæµÄŠ”ĖÕ“ņ£¬³ä·Ö·“Ó¦ŗó²śÉśĘųĢåŌŚ±ź×¼×“æöĻĀĖłÕ¼µÄĢå»żŹĒ
£Ø3£©³£ĪĀŹ±£¬BaSO4µÄKsp£½1.08”Į10-10,ĻÖ½«µČĢå»żµÄBaCl2ČÜŅŗÓė2.0”Į10-3mol/lµÄNa2SO4
ČÜŅŗ»ģŗĻ”£ČōŅŖÉś³ÉBaSO4³Įµķ£¬BaCl2ČÜŅŗµÄ×īŠ”ÅضČĪŖ______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©Ä³Ń§ÉśÓĆ0.1000mol”¤L£1±ź×¼NaOHČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖį£¬Ęä²Ł×÷æÉ·Ö½āĪŖŅŌĻĀ¼ø²½£ŗ
AŅĘČ”25.00mL“ż²āŃĪĖįČÜŅŗ×¢Čė½ą¾»µÄ׶ŠĪĘæÖŠ£¬²¢¼ÓČė2”«3µĪ·ÓĢŖČÜŅŗ
BÓƱź×¼NaOHČÜŅŗČóĻ“µĪ¶Ø¹Ü2”«3“Ī
C°ŃŹ¢ÓŠ±ź×¼NaOHČÜŅŗµÄ¼īŹ½µĪ¶Ø¹Ü¹Ģ¶ØŗĆ£¬¼·Ń¹²£Į§Ö飬Ź¹µĪ¶Ø¹Ü¼ā×ģ³äĀśČÜŅŗ
DČ”±ź×¼NaOHČÜŅŗ×¢Čė¼īŹ½µĪ¶Ø¹Üµ½”°0”±æĢ¶ČŅŌÉĻ2”«3cm
E£®µ÷½ŚŅŗĆęÖĮ”°0”±»ņ”°0”±æĢ¶ČŅŌĻĀ£¬¼ĒĻĀ¶ĮŹż
F£®°Ń׶ŠĪĘæ·ÅŌŚµĪ¶Ø¹ÜµÄĻĀĆę£¬ÓƱź×¼NaOHČÜŅŗµĪ¶Øµ½ÖÕµć£¬²¢¼ĒĻĀµĪ¶Ø¹ÜŅŗĆęµÄ¶ĮŹż
£Ø1£©ĻĀĶ¼ÖŠŹōÓŚ¼īŹ½µĪ¶Ø¹ÜµÄ £ØŃ”Ģī£ŗ”°¼×”±”¢”°ŅŅ”±£©”£
£Ø2£©ÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ £ØĢī×ÖÄøŠņŗÅ£©”£
£Ø3£©ÉĻŹöB²½²Ł×÷µÄÄæµÄŹĒ ”£
£Ø4£©ÅŠ¶Ļµ½“ļµĪ¶ØÖÕµćµÄŹµŃéĻÖĻóŹĒ ”£
£Ø5£©ÉĻŹöA²½²Ł×÷Ö®Ē°£¬ČōĻČÓĆ“ż²āČÜŅŗČóĻ“׶ŠĪĘ棬Ōņ¶ŌµĪ¶Ø½į¹ūµÄÓ°ĻģŹĒ £ØĢī”°Ę«“ó”±»ņ”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±£©”£
£Ø6£©ČōĘ½ŠŠŹµŃéČż“Ī£¬¼ĒĀ¼µÄŹż¾ŻČēĻĀ±ķ
| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗµÄĢå»ż(/mL) | ±ź×¼NaOHČÜŅŗµÄĢå»ż | |
| µĪ¶ØĒ°¶ĮŹż(/mL) | µĪ¶Øŗó¶ĮŹż(/mL) | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
“ÖCuOŹĒ½«¹¤Ņµ·ĻĶ”¢·ĻĶŗĻ½šµČøßĪĀ±ŗÉÕ¶ų³ÉµÄ£¬ŌÓÖŹÖ÷ŅŖŹĒĢśµÄŃõ»ÆĪļ¼°ÄąÉ³”£ŅŌ“ÖCuOĪŖŌĮĻÖʱøµØ·ÆµÄÖ÷ŅŖĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖFe3+”¢Fe2+ ”¢Cu2+×Ŗ»ÆĪŖĻąÓ¦ĒāŃõ»ÆĪļŹ±£¬æŖŹ¼³ĮµķŗĶ³ĮµķĶźČ«Ź±µÄpHČēĻĀ±ķ£ŗ
| | Fe3+ | Fe2+ | Cu2+ |
| æŖŹ¼³ĮµķŹ±µÄpH | 2.7 | 7.6 | 5.2 |
| ĶźČ«³ĮµķŹ±µÄpH | 3.7 | 9.6 | 6.4 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com