
Ϊ�˺������û�ѧ��ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
(1)ʵ���ã�5 g�״��������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5 kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ��___________________________________________��
(2)����̬��̬ԭ���γ�1 mol��ѧ���ͷŵ���������м��ܡ��ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ���Ķ��Ѻ�������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ���������֪��ӦN2(g)��3H2(g)2NH3(g)����H��a kJ��mol��1���Ը��ݱ������м������ݹ���a����ֵ��
________________________________________________________________________��
| ��ѧ�� | H��H | N��H | N��N |
| ����/kJ��mol��1 | 436 | 391 | 945 |
(3)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��
C(ʯī��s)��O2(g)===CO2(g)
��H1����393.5 kJ��mol��1��
2H2(g)��O2(g)===2H2O(l)
��H2����571.6 kJ��mol��1��
2C2H2(g)��5O2(g)===4CO2(g)��2H2O(l)
��H3����2 599 kJ��mol��1��
���ݸ�˹���ɣ�����298 Kʱ��C(ʯī��s)��H2(g)����1 mol C2H2(g)��Ӧ���ʱ䣺________________________________________________________________________��
�𰸡�(1)2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)
��H����1 452.8 kJ��mol��1
(2)��93
(3)��H��226.7 kJ��mol��1
������(1)5 g CH3OH(l) ���ȼ������CO2(g)��H2O(l)���ų�113.5 kJ����������1 mol CH3OH���ȼ��ʱ���ų�������Ϊ ��113.5 kJ��726.4 kJ������ȼ�յ��Ȼ�ѧ����ʽΪCH3OH(l)��
��113.5 kJ��726.4 kJ������ȼ�յ��Ȼ�ѧ����ʽΪCH3OH(l)��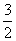 O2(g)===CO2(g)��2H2O(l)����H����726.4 kJ��mol��1����2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)����H����1 452.8 kJ��mol��1��
O2(g)===CO2(g)��2H2O(l)����H����726.4 kJ��mol��1����2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)����H����1 452.8 kJ��mol��1��
(2)��H����Ӧ��ļ���֮�ͣ�������ļ���֮�ͣ�3��436 kJ��mol��1��945 kJ��mol��1��6��391 kJ��mol��1����93 kJ��mol��1����a��ֵΪ��93��
(3)������֪��������������Ӧ���ʱ䣺
2C(ʯī��s)��H2(g)===C2H2(g)����H���ɸ�˹���ɣ�������Ȼ�ѧ����ʽ�Ӻϣ�(�١�4���ڣ���)/2�ã�
2C(ʯī��s)��H2(g)��C2H2(g)����H��2��H1��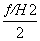 ��
��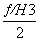 ��H��[2��(��393.5)��
��H��[2��(��393.5)��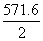 ��
��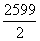 ] kJ��mol��1��226.7 kJ��mol��1��
] kJ��mol��1��226.7 kJ��mol��1��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����л������������Ϊ��Ǧ���͵Ŀ����������ʽ��Ϊ88.0����C����������Ϊ0.682����H����������Ϊ0.136��
(1)��ȷ���û�����ķ���ʽ_____________________________________________________��
(2)��������ͺ˴Ź���������ʾ�÷�������4��������д����ṹ��ʽ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1���ij����������ȫȼ�����ɵ�CO2��ˮ������1���(��ͬ��ͬѹ�²ⶨ)��0.1 mol������ȫȼ�յIJ���ȫ������ʯ�����գ���ʯ������ 51.4 g���Խ��
(1)������ķ���ʽ________________________________________________________��
(2)��������һ�ȴ���ֻ��һ�֣���д�������Ľṹ��ʽ����ϵͳ����������
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�γɽ�Լ��Դ�ͱ�����̬�����IJ�ҵ�ṹ����������Ȼ��г��չ����Ҫ��֤������Ϊ������Ϊ���������һ��֤����(����)
A������̫���ܡ�ˮ�ܡ����ܵ�����Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ��
B���о���ú�������¼�������߲��������㹤ҵ�����Ŀ��ٷ�չ
C����ũ���ƹ�ʹ������
D��������Դ���ġ�������Դ���ظ�ʹ�ú���Դ��ѭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪H2(g)��C2H4(g)��C2H5OH(l)�ı�ȼ���ȷֱ���285.8 kJ��mol��1��1 411.0 kJ��mol��1��1 366.8 kJ��mol��1������C2H4(g)��H2O(l)��Ӧ����C2H5OH(l)�Ħ�HΪ(����)
A����44.2 kJ��mol��1
B��44.2 kJ��mol��1
C����330 kJ��mol��1
D��330 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���Ԫ�ص�������ȷ���� (����)��
A����һ�����ܣ�B>Al>Ga
B���縺�ԣ�F>N>O
C��������ۣ�F>S>Si
D��ԭ�Ӱ뾶��P>N>C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪Ԫ�����ڱ��й���18���У���ͼʵ�߱�ʾԪ�����ڱ��ı߽磬�������Ų����ɰ����ڱ�����Ϊs����p����d����ds���ȡ���ds���⣬�����������ƾ�������ԭ�����������ӵ��ܼ�������������
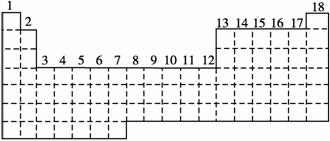
(1)����ͼ����ʵ����s����p����d����ds���ı߽��ߣ�����Ƿ�����
(2)�е�ͬѧ�����ֻ��ֵ���ʾ����Ϊd����6��7���еIJ���Ԫ�ؿ���������
һ��������ΪӦ����________����
(3)����Ԫ�����ڱ��б��4s����������Ԫ��(��Ԫ�ط��ű�ʾ)��
(4)�����õ����Ų������֪ʶ����Fe3����Fe2���ȶ���ԭ��______________��
(5)���ſ�ѧ�����ķ�չ���������µ�Ԫ�ر����֡����ѵ���������������Ԫ
�����ڱ��������Ų�________��Ԫ�ء�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���M�Ľṹ��ʽ��ͼ��ʾ��
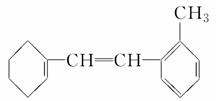
(1)�л���M�ı����ϵ�һ�ȴ�����________�֡�
(2)1 mol M��������ˮ��ϣ�����Br2�����ʵ���Ϊ________mol��
(3)1 mol M������H2�ӳɣ�����H2________ mol��
(4)�����й�M��˵���в���ȷ����________��
A���ڴ����������£�M����Һ�巢��ȡ����Ӧ
B��Mʹ��ˮ��ɫ��ԭ������ϩʹ��ˮ��ɫ��ԭ����ͬ
C��M��ʹ����KMnO4��Һ��ɫ
D��M�ͼױ���Ϊͬϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NaBr��NaI��Na2SO3�Ļ��Һ�У�ͨ����������������Һ���ɲ�������գ��õ�����ʣ�����ʵ���ɿ�����(����)
A��NaCl��Na2SO4 B��NaCl��NaBr��Na2SO4 C��NaCl��Na2SO4��I2 D��NaCl��NaI��Na2SO4
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com