

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | SO42- | Mg2+ | Fe3+ | Na+ | Cl- |
| Ũ�ȣ�mol/L�� | a | 0.05 | 0.10 | 0.50 | 0.58 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | SO42- | Mg2+ | Fe3+ | Na+ | Cl- |
| Ũ�ȣ�mol/L�� | a | 0.05 | 0.10 | 0.50 | 0.58 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
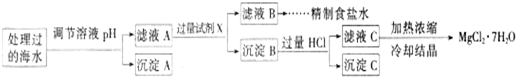
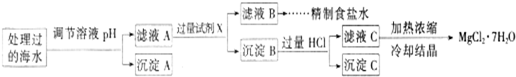
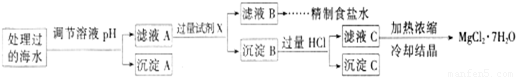
Ŀǰ��������(Na2FeO4)���㷺Ӧ����ˮ���������и�Ч�������ŵ㡣
(1)�ڴ���ˮ�Ĺ����У�Na2FeO4��ɱ������������ˮ�����ã���˵��Ӧ����Na2FeO4����Щ���ʣ�__________________________________________________________________________
(2) ij�غ�ˮ��Ʒ��Na2FeO4�������������Ӽ���Ũ�����±���ʾ(H����OH��δ�г�)��
| ���� | SO | Mg2�� | Fe3�� | Na�� | Cl�� |
| Ũ��(mol/L) | a | 0.05 | 0.10 | 0.50 | 0.58 |
�����£�ȡһ������Na2FeO4�������ĺ�ˮΪԭ���Ʊ�����ʳ��ˮ��MgCl2��7H2O���������£�

Ksp[Fe(OH)3]��1.0��10��38��Ksp[Mg(OH)2]��5.0��10��12��������������Һ����ı仯���Բ��ơ�
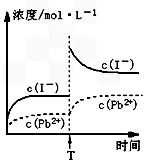
�� �����е�a________0.16(�<������>������)��
�� ����A�����Ϊ________(�ѧʽ)���ڵ�����ҺpHʱ��������Ӧ���ڵ�pH�ķ�Χ��________��
�� ����Ĺ����Լ�XΪ____________________(�ѧʽ)��
�� �������HCl������Ϊ_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��Ϫһ�и������ϣ��������¿���ѧ�Ծ��������棩 ���ͣ������
| ���� | SO42- | Mg2+ | Fe3+ | Na+ | Cl- |
| Ũ�ȣ�mol/L�� | a | 0.05 | 0.10 | 0.50 | 0.58 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com