
½¹ŃĒĮņĖįÄĘ(Na2S2O5)ŹĒ³£ÓƵď³Ę·æ¹Ńõ»Æ¼ĮÖ®Ņ»”£Ä³ŃŠ¾æŠ”×é½ųŠŠČēĻĀŹµŃé£ŗ
ŹµŃéŅ»””½¹ŃĒĮņĖįÄʵÄÖĘČ”
²ÉÓĆĻĀĶ¼×°ÖĆ(ŹµŃéĒ°ŅŃ³ż¾”×°ÖĆÄŚµÄæÕĘų)ÖĘČ”Na2S2O5”£×°ÖĆ¢ņÖŠÓŠNa2S2O5¾§ĢåĪö³ö£¬·¢ÉśµÄ·“Ó¦ĪŖNa2SO3£«SO2===Na2S2O5”£
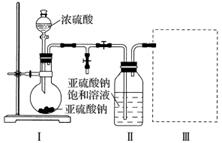
(1)×°ÖĆ¢ńÖŠ²śÉśĘųĢåµÄ»Æѧ·½³ĢŹ½ĪŖ__________________________________________”£
(2)ŅŖ“Ó×°ÖĆ¢ņÖŠ»ńµĆŅŃĪö³öµÄ¾§Ģ壬æɲÉČ”µÄ·ÖĄė·½·ØŹĒ________________________________________________________________________
________________________________________________________________________ӣ
(3)×°ÖĆ¢óÓĆÓŚ“¦ĄķĪ²Ęų£¬æÉŃ”ÓƵÄ×īŗĻĄķ×°ÖĆ(¼Š³ÖŅĒĘ÷ŅŃĀŌČ„)ĪŖ__________(ĢīŠņŗÅ)”£
ŹµŃ鶞””½¹ŃĒĮņĖįÄʵĊŌÖŹ
Na2S2O5ČÜÓŚĖ®¼“Éś³ÉNaHSO3”£
(4)Ö¤Ć÷NaHSO3ČÜŅŗÖŠHSO µÄµēĄė³Ģ¶Č“óÓŚĖ®½ā³Ģ¶Č£¬æɲÉÓƵďµŃé·½·ØŹĒ_____(ĢīŠņŗÅ)”£
µÄµēĄė³Ģ¶Č“óÓŚĖ®½ā³Ģ¶Č£¬æɲÉÓƵďµŃé·½·ØŹĒ_____(ĢīŠņŗÅ)”£
a£®²ā¶ØČÜŅŗµÄpH
b£®¼ÓČėBa(OH)2ČÜŅŗ
c£®¼ÓČėŃĪĖį
d£®¼ÓČėĘ·ŗģČÜŅŗ
e£®ÓĆĄ¶É«ŹÆČļŹŌÖ½¼ģ²ā
(5)¼ģŃéNa2S2O5¾§ĢåŌŚæÕĘųÖŠŅѱ»Ńõ»ÆµÄŹµŃé·½°øŹĒ__________”£
“š°ø””(1)Na2SO3£«H2SO4(ÅØ)===Na2SO4£«SO2”ü£«H2O(»ņNa2SO3£«2H2SO4(ÅØ)===2NaHSO4£«SO2”ü£«H2O)
(2)¹żĀĖ””(3)d””(4)ae
(5)ȔɣĮæNa2S2O5¾§ĢåÓŚŹŌ¹ÜÖŠ£¬¼ÓŹŹĮæĖ®Čܽā£¬µĪ¼Ó×ćĮæŃĪĖį£¬Õńµ“£¬ŌŁµĪČėĀČ»Æ±µČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É
½āĪö””(1)×°ÖĆ¢ńÖŠ·¢ÉśµÄŹĒŹµŃéŹŅÖʱøSO2µÄ·“Ó¦£¬²ÉÓƵÄŌĄķŹĒĒæĖįÖʱøČõĖį£¬¹Ź»Æѧ·½³ĢŹ½ĪŖNa2SO3£«H2SO4(ÅØ)===Na2SO4£«SO2”ü£«H2O”£
(2)½«½¹ŃĒĮņĖįÄĘ¾§Ģå“ÓČÜŅŗÖŠ·ÖĄė³öĄ“Ó¦øĆ²ÉČ”¹żĀĖµÄ·½·Ø”£
(3)ŹµŃé²śÉśµÄĪ²ĘųÖ÷ŅŖŹĒSO2ĘųĢ壬a×°ÖĆŹĒĆܱյĻ·¾³£¬SO2Ķز»½ųČ„£¬b×°ÖĆÖŠŹ³ŃĪĖ®ĪüŹÕSO2µÄŠ§¹ū²»ČēdŗĆ£¬ĒŅŅ×ŅżĘšµ¹Īü£¬c×°ÖĆÅØH2SO4²»ĪüŹÕSO2£¬SO2ŹĒŅ»ÖÖĖįŠŌŃõ»ÆĪļ£¬Ņņ“ĖÓĆNaOHČÜŅŗĪüŹÕ×īŗĆ£¬ĒŅd×°ÖĆ»¹²»Ņ×ŅżĘšµ¹Īü”£
(4)HSO ·¢ÉśµēĄė£ŗHSO
·¢ÉśµēĄė£ŗHSO ??H£«£«SO
??H£«£«SO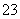 £¬Ķ¬Ź±»į·¢ÉśĖ®½ā£ŗHSO
£¬Ķ¬Ź±»į·¢ÉśĖ®½ā£ŗHSO £«H2O??H2SO3£«OH££¬ČōHSO
£«H2O??H2SO3£«OH££¬ČōHSO µÄµēĄė“óÓŚHSO
µÄµēĄė“óÓŚHSO µÄĖ®½ā£¬ŌņČÜŅŗĻŌĖįŠŌ£¬¹Ź“š°øa”¢eÕżČ·”£
µÄĖ®½ā£¬ŌņČÜŅŗĻŌĖįŠŌ£¬¹Ź“š°øa”¢eÕżČ·”£
(5)Na2S2O5ÖŠSŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ£«4¼Ū£¬Ņņ“ĖŌŚæÕĘųÖŠŅ×±»Ńõ»ÆĪŖ£«6¼ŪµÄSO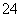 £¬Ņņ“Ė±¾Ģā¾Ķ×Ŗ»ÆĪŖSO
£¬Ņņ“Ė±¾Ģā¾Ķ×Ŗ»ÆĪŖSO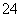 µÄ¼ģŃ飬¹ŹČ”ÉŁĮæ¹ĢĢåĻČ¼ÓŃĪĖįĖį»Æ£¬ÅųżĘäĖūĄė×ÓµÄøÉČÅ£¬ŌŁ¼ÓBaCl2ČÜŅŗ£¬æ“ŹĒ·ńÓŠ°×É«³Įµķ²śÉś¼“æÉ”£
µÄ¼ģŃ飬¹ŹČ”ÉŁĮæ¹ĢĢåĻČ¼ÓŃĪĖįĖį»Æ£¬ÅųżĘäĖūĄė×ÓµÄøÉČÅ£¬ŌŁ¼ÓBaCl2ČÜŅŗ£¬æ“ŹĒ·ńÓŠ°×É«³Įµķ²śÉś¼“æÉ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠµē×ÓÅŲ¼Ź½ÖŠ£¬Ō×Ó“¦ÓŚ¼¤·¢Ģ¬µÄŹĒ(””””)
A£®1s22s22p5
B£®1s22s22p43s2
C£®1s22s22p63s23p63d54s1
D£®1s22s22p63s23p63d34s2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŖĖŲÖÜĘŚĀÉŹĒ20ŹĄ¼ĶæĘѧ¼¼Źõ·¢Õ¹µÄÖŲŅŖĄķĀŪŅĄ¾ŻÖ®Ņ»”£ŅŃÖŖA”¢B”¢C”¢D”¢EĪåÖÖŌŖĖŲ¶¼ŹĒŌŖĖŲÖÜĘŚ±ķÖŠĒ°20ŗÅŌŖĖŲ£¬A”¢B”¢C”¢DĖÄÖÖŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķ(³¤Ź½)ÖŠµÄĻą¶ŌĪ»ÖĆČēĻĀĶ¼ĖłŹ¾£¬B”¢C”¢DµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļĮ½Į½»ģŗĻ£¬¾łÄÜ·¢Éś·“Ӧɜ³ÉŃĪŗĶĖ®£¬EŌŖĖŲŌ×ÓŠņŹż¾ł“óÓŚA”¢B”¢C”¢DŌŖĖŲ£¬ĒŅ²»ÓėA”¢B”¢C”¢DŌŖĖŲĪ»ÓŚĶ¬Ö÷×唣

øł¾ŻŅŌÉĻŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÉĻŹöĪåÖÖŌŖĖŲÖŠµēøŗŠŌ×ī“óµÄŹĒ________”£(ĢīĻą¹ŲŌŖĖŲµÄŌŖĖŲ·ūŗÅ)
(2)ĒėŠ“³öDµÄµē×ÓÅŲ¼Ź½£ŗ____________________________”£
(3)AŗĶDµÄĒā»ÆĪļÖŠ£¬·Šµć½ĻøߵďĒ________(ĢīĻą¹ŲĪļÖŹµÄ·Ö×ÓŹ½)£»ĘäŌŅņŹĒ
____________________________________ӣ
(4)AŗĶEæÉ×é³ÉĄė×Ó»ÆŗĻĪļ£¬Ę侧°ū(ŌŚ¾§ĢåÖŠ¾ßÓŠ“ś±ķŠŌµÄ×īŠ”ÖŲø“µ„ŌŖ)½į¹¹ČēĻĀĶ¼ĖłŹ¾£¬ŃōĄė×ÓĪ»ÓŚøĆÕż·½ĢåµÄ¶„µć»ņĆęŠÄ£»ŅõĄė×Ó¾łĪ»ÓŚŠ”Õż·½ĢåÖŠŠÄ”£øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ____________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
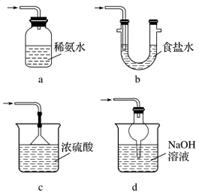
ĮņĖįŹĒ֊ѧ»ÆѧŹµŃéŹŅµÄ³£¼ūŅ©Ę·£¬ĘäŠŌÖŹÓŠ¢ŁĖįŠŌ””¢ŚĪüĖ®ŠŌ””¢ŪĶŃĖ®ŠŌ””¢ÜĒæŃõ»ÆŠŌ””¢Ż“ß»Æ×÷ÓĆ£¬Ēė½«ŠņŗÅĢīŌŚĻąÓ¦µÄŗįĻßÉĻ£ŗ
(1)ŠæŗĶĻ”H2SO4ÖĘH2________£»
(2)ÅØĮņĖį×÷øÉŌļ¼Į________£»
(3)ÅØĮņĖįÓėÕįĢĒµÄĢæ»ÆŹµŃé(ŗŚĆę°üŹµŃé)________£»
(4)ŹµŃéŹŅÓĆŅŅ“¼ŗĶ±ł“×ĖįÖĘČ”ŅŅĖįŅŅõ„________£»
(5)ŅŅĖįŅŅõ„µÄĖ®½ā________£»
(6)ĻĖĪ¬ĖŲµÄĖ®½ā________£»
(7)ÅØĮņĖįÓėĶµÄ·“Ó¦________£»
(8)ÅØĮņĖįŹ¹ŹŖČóŹÆČļŹŌÖ½±äŗģ£¬ŗóĄ“ÓÖ±äŗŚ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĶŃĮņ¼¼ŹõÄÜÓŠŠ§æŲÖĘSO2¶ŌæÕĘųµÄĪŪČ¾”£
(1)ĻņĆŗÖŠ¼ÓČėŹÆ»ŅŹÆæɼõÉŁČ¼ÉÕ²śĪļÖŠSO2µÄŗ¬Į棬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________________________ ”£
(2)ŗ£Ė®³ŹČõ¼īŠŌ£¬Ö÷ŅŖŗ¬ÓŠNa£«”¢K£«”¢Ca2£«”¢Mg2£«”¢Cl£”¢SO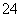 ”¢Br£”¢HCO
”¢Br£”¢HCO µČ”£ŗ¬SO2µÄŃĢĘųæÉĄūÓĆŗ£Ė®ĶŃĮņ£¬Ę乤ŅÕĮ÷³ĢČēĶ¼ĖłŹ¾£ŗ
µČ”£ŗ¬SO2µÄŃĢĘųæÉĄūÓĆŗ£Ė®ĶŃĮņ£¬Ę乤ŅÕĮ÷³ĢČēĶ¼ĖłŹ¾£ŗ

¢ŁĻņĘŲĘų³ŲÖŠĶØČėæÕĘųµÄÄæµÄŹĒ______________________________________________”£
¢ŚĶØČėæÕĘųŗó£¬ĘŲĘų³ŲÖŠµÄŗ£Ė®ÓėĢģČ»ŗ£Ė®Ļą±Č£¬ÅضČÓŠĆ÷ĻŌ²»Ķ¬µÄĄė×ÓŹĒ______(Ģī×ÖÄø)”£
a£®Cl£ b£®SO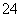 c£®Br£ d£®HCO
c£®Br£ d£®HCO
(3)ÓĆNaOHČÜŅŗĪüŹÕŃĢĘųÖŠµÄSO2£¬½«ĖłµĆµÄNa2SO3ČÜŅŗ½ųŠŠµē½ā£¬æɵƵ½NaOH£¬Ķ¬Ź±µĆµ½H2SO4£¬ĘäŌĄķČēĶ¼ĖłŹ¾”£(µē¼«²ÄĮĻĪŖŹÆÄ«)

¢ŁĶ¼ÖŠa¼«Į¬½ÓµēŌ“µÄ______(Ģī”°Õż”±»ņ”°øŗ”±)¼«£¬CæŚĮ÷³öµÄĪļÖŹŹĒ________”£
¢ŚSO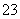 ·ÅµēµÄµē¼«·“Ó¦ĪŖ________________________”£
·ÅµēµÄµē¼«·“Ó¦ĪŖ________________________”£
¢Ūµē½ā¹ż³ĢÖŠŅõ¼«Ēų¼īŠŌĆ÷ĻŌŌöĒ棬ÓĆĘ½ŗāŅĘ¶ÆµÄŌĄķ½āŹĶ¼īŠŌŌöĒæµÄŌŅņ£ŗ________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĻÖĻó»ņŹĀŹµæÉÓĆĶ¬Ņ»ŌĄķ½āŹĶµÄŹĒ(””””)
A£®ÅØĮņĖįŗĶÅØŃĪĖį³¤ĘŚ±©Ā¶ŌŚæÕĘųÖŠÅØ¶Č½µµĶ
B£®SO2ŗĶFeSO4ČÜŅŗŹ¹ĖįŠŌøßĆĢĖį¼ŲµÄ×ĻÉ«ĶŹČ„
C£®ĘÆ°×·ŪŗĶĖ®²£Į§³¤ĘŚ±©Ā¶ŌŚæÕĘųÖŠ±äÖŹ
D£®ŃĒĮņĖįÄĘČÜŅŗŗĶĀČ»ÆĀĮČÜŅŗŌŚæÕĘųÖŠÕōøɲ»ÄܵƵ½¶ŌÓ¦µÄČÜÖŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
֊ѧ»Æѧ֊¼øÖÖ³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼(²æ·Ö²śĪļĪ“ĮŠ³ö)”£AŹĒŅ»ÖÖ½šŹōµ„ÖŹ£¬DŹĒŅ»ÖÖ·Ē½šŹō¹ĢĢåµ„ÖŹ”£

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)A”¢CµÄ»ÆѧŹ½·Ö±šĪŖA__________£¬C__________”£
(2)FµÄÅØČÜŅŗÓėA·“Ó¦¹ż³ĢÖŠ£¬FĢåĻֵĊŌÖŹÓėĻĀĮŠ·“Ó¦ÖŠH2SO4ĢåĻֵĊŌÖŹĶźČ«ĻąĶ¬µÄŹĒ________(Ģī×ÖÄø)”£
A£®C£«2H2SO4(ÅØ)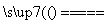 CO2”ü£«2SO2”ü£«2H2O
CO2”ü£«2SO2”ü£«2H2O
B£®Fe£«H2SO4===FeSO4£«H2”ü
C£®Cu£«2H2SO4(ÅØ)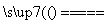 CuSO4£«SO2”ü£«2H2O
CuSO4£«SO2”ü£«2H2O
D£®FeO£«H2SO4===FeSO4£«H2O
(3)Š“³ö·“Ó¦E£«H2O2”Ŗ”śFµÄ»Æѧ·½³ĢŹ½£ŗ__________________________________”£
(4)Čō·“Ó¦F£«D”Ŗ”śE×ŖŅʵē×ÓŹżĪŖ6.02”Į1023£¬ŌņĻūŗÄDµÄÖŹĮæĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A£«”¢B£«”¢C£”¢D”¢EĪåÖÖĮ£×Ó(·Ö×Ó»ņĄė×Ó)ÖŠ£¬ĆæøöĮ£×Ó¾łÓŠ10øöµē×Ó£¬ŅŃÖŖ£ŗ
¢ŁA£«£«C£===D£«E”ü£»¢ŚB£«£«C£===2D”£
Ēė»Ų“š£ŗ
(1)C£Ąė×ӵĵē×ÓŹ½ŹĒ________________”£
(2)A£«Ąė×ÓÖŠµÄ¼ü½ĒĪŖ______________”£
(3)·Ö±šŠ“³öA£«ŗĶD·“Ó¦”¢B£«ŗĶE·“Ó¦µÄĄė×Ó·½³ĢŹ½____________”¢____________”£
(4)³żD”¢EĶā£¬ĒėŌŁŠ“³öĮ½ÖÖŗ¬10øöµē×ӵķÖ×ӵķÖ×ÓŹ½____________”£
(5)³żA£«”¢B£«Ķā£¬ĒėŌŁŠ“³öĮ½ÖÖŗ¬10øöµē×ÓµÄŃōĄė×Ó________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼µÄ×°ÖĆÖŠ£¬øÉŌļÉÕĘæÄŚŹ¢ÓŠÄ³ÖÖĘųĢ壬ÉÕ±ŗĶµĪ¹ÜÄŚŹ¢·ÅijÖÖČÜŅŗ”£¼·Ń¹µĪ¹ÜµÄ½ŗĶ·£¬ĻĀĮŠÓėŹµŃéŹĀŹµ²»Ļą·ūµÄŹĒ(””””)
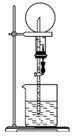
A£®CO2(NaHCO3ČÜŅŗ)/ĪŽÉ«ÅēČŖ
B£®NH3(H2OÖŠŗ¬·ÓĢŖ)/ŗģÉ«ÅēČŖ
C£®H2S(CuSO4ČÜŅŗ)/ŗŚÉ«ÅēČŖ
D£®HCl(AgNO3ČÜŅŗ)/°×É«ÅēČŖ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com