
��10�֣�X��Y��Z��R��T��ԭ��������������Ķ���������Ԫ�أ�X��R�����ڱ��е����λ�����ұ���ʾ��XԪ��������ϼ۵ľ���ֵ������ԭ�ӵ�������������Z�ǵؿ��к������Ľ���Ԫ�ء�

��1��X�����������ĵ���ʽ��_________��Ԫ��T�����ڱ��е�λ����________________��
��2��Z��T�γɵĻ������ڳ�ʪ�Ŀ�����ð��ɫ�������÷�Ӧ�Ļ�ѧ����ʽΪ________________��
��3��Y�������̬�⻯���ˮ��Һ�Լ��ԡ������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ________________��
��4����֪��X��R�γɵ�Һ̬������XR2��ȼ������1075 kj/mol����д����ʾ��ȼ���ȵ��Ȼ�ѧ����ʽ________________
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| X | ||||
| R |
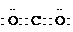
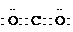
 Al��OH��3+3HCl��
Al��OH��3+3HCl�� Al��OH��3+3HCl��
Al��OH��3+3HCl���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�X��Y��Z��R��T��ԭ��������������Ķ���������Ԫ�أ�X��R�����ڱ��е����λ�����ұ���ʾ��XԪ��������ϼ۵ľ���ֵ������ԭ�ӵ�������������Z�ǵؿ��к������Ľ���Ԫ�ء�
��1��X�����������ĵ���ʽ��_________��Ԫ��T�����ڱ��е�λ����________________��
��2��Z��T�γɵĻ������ڳ�ʪ�Ŀ�����ð��ɫ�������÷�Ӧ�Ļ�ѧ����ʽΪ________________��
��3��Y�������̬�⻯���ˮ��Һ�Լ��ԡ������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ________________��
��4����֪��X��R�γɵ�Һ̬������XR2��ȼ������1075kj/mol����д����ʾ��ȼ���ȵ��Ȼ�ѧ����ʽ________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011--2012ѧ�����ʡ�����ص���ѧ������һ���������⻯ѧ�Ծ� ���ͣ������
��10�֣�X��Y��Z��R��T��ԭ��������������Ķ���������Ԫ�أ�X��R�����ڱ��е����λ�����ұ���ʾ��XԪ��������ϼ۵ľ���ֵ������ԭ�ӵ�������������Z�ǵؿ��к������Ľ���Ԫ�ء�
��1��X�����������ĵ���ʽ��_________��Ԫ��T�����ڱ��е�λ����________________��
��2��Z��T�γɵĻ������ڳ�ʪ�Ŀ�����ð��ɫ�������÷�Ӧ�Ļ�ѧ����ʽΪ________________��
��3��Y�������̬�⻯���ˮ��Һ�Լ��ԡ������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ________________��
��4����֪��X��R�γɵ�Һ̬������XR2��ȼ������1075 kj/mol����д����ʾ��ȼ���ȵ��Ȼ�ѧ����ʽ________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�ij�����������У�����������ϣ����л�ѧ�Ծ��������棩 ���ͣ������
| X | ||||
| R |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com