

| | �Ƿ���ȷ | �������� |
| ѧ���Ĺ۵� | | |
| ѧ���ҵĹ۵� | | |
| ѧ�����Ĺ۵� | | |
A�� |
B�� |
C�� |
D�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
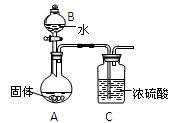
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
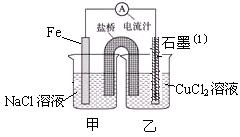
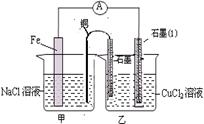
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
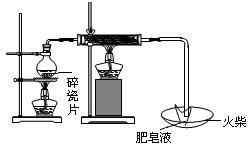
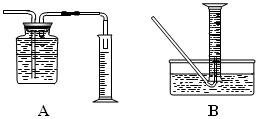
| | ���� | ��������� |
| ���ܢ� | | |
| ���ܢ� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �ձ���� | �� | �� | �� |
| �����Լ� | ����0.1g | ����0.1g��0.1mol/LH2SO43mL | 0.1mol/LH2SO43mL |
| ��ɫ����ʱ��(��) | 1�� | 4������ | 8������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��m��50��18��6.02��1023 |
B�� ��6.02��1023 ��6.02��1023 |
C�� ��6.02��1023 ��6.02��1023 |
D��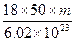 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��SO2ͨ������KMnO4��Һ�� | ֤��SO2����Ư���� |
| B | ��Cl2ͨ��NaBr��Һ�У�Ȼ�����CCl4�������� | �Ƚ��������������ǿ�� |
| C | ��ͭƬ�ֱ���Ũ��ϡ���ᷴӦ | ̽��Ũ��ϡ���������Ե����ǿ�� |
| D | ��ʢ��20g���ǵ��ձ��м��뼸��ˮ��������ȡ��ټ�������Ũ���ᣬѸ�ٽ��衣 | ̽��Ũ�������ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ˼����������һ��Һ�������ܿ�����һ������ɫ���ţ����ܿ�д�˻��š�С�źͱ������õ���Һ�ֱ�����ǣ� ����
˼����������һ��Һ�������ܿ�����һ������ɫ���ţ����ܿ�д�˻��š�С�źͱ������õ���Һ�ֱ�����ǣ� ���� A��ǰ������ɫʯ����Һ��������ϡ���� | B��ǰ����NaOHϡ��Һ��������ϡ���� |
| C��ǰ����Ũ���ᣬ��������ɫʯ����Һ | |
| D��ǰ���������������ǵ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com