
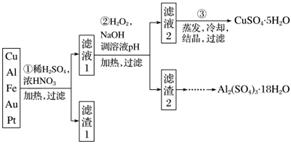
��Ϣʱ�������Ĵ������������Ի��������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70% Cu��25% Al��4% Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�
��ش��������⣺
(1)�ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ____________________________________________
________________________________________________________________________��
�õ�����1����Ҫ�ɷ�Ϊ____________��
(2)�ڢڲ���H2O2��������______________��ʹ��H2O2���ŵ���______________������ҺpH��Ŀ����ʹ______________���ɳ�����
(3)�õڢ۲�����CuSO4·5H2O�Ʊ���ˮCuSO4�ķ�����___________________________��
(4)������2��ȡAl2(SO4)3·18H2O ��̽��С����������ַ�����
�ף�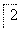
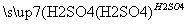
 ,��������ȴ���ᾧ������
,��������ȴ���ᾧ������
Al2(SO4)3·18H2O
�ң�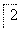
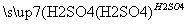

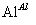

,��������ȴ���ᾧ������Al2(SO4)3·18H2O
����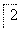
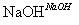

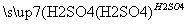

,��������ȴ���ᾧ������Al2(SO4)3·18H2O
�������ַ����У�________���������У�ԭ����________________________________
________________________________________________________________________��
��ԭ�������ʽǶȿ��ǣ�__________������������
�𰸡�(1)Cu��4H����2NO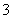
 Cu2����2NO2����2H2O��3Cu��8H����2NO
Cu2����2NO2����2H2O��3Cu��8H����2NO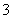
 3Cu2����2NO����4H2O��Au��Pt
3Cu2����2NO����4H2O��Au��Pt
(2)��Fe2������ΪFe3�������������ʣ��Ի�������Ⱦ��Fe3����Al3����(3)������ˮ
(4)�ס����ò�Ʒ�к��н϶�Fe2(SO4)3���ʡ���
�����������Ʊ�·�߿�֪��Cu��Al��Fe��Au��Pt�Ļ�����м���ϡH2SO4��Ũ���ᣬ����Խ�Cu��Al��Fe�ܽ⣬Au��Pt���ܽ⣬��������1����Ҫ�ɷ�ΪAu��Pt����Һ1�к���Cu��Fe��Al�����ӡ�������Һ2��CuSO4·5H2O��֪����Һ2ΪCuSO4��Һ������2�к���Fe(OH)3��Al(OH)3��
(1)�ڢٲ���Cu���ᷢ���ķ�ӦΪCu��Ũ����ķ�Ӧ��Cu��4H����2NO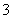
 Cu2����2NO2����2H2O��Ũ������ϡ����Ļ�Ϲ����������ϡ�������ķ�ӦҲ����Ϊ3Cu��8H����2NO
Cu2����2NO2����2H2O��Ũ������ϡ����Ļ�Ϲ����������ϡ�������ķ�ӦҲ����Ϊ3Cu��8H����2NO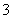
 3Cu2����2NO����4H2O�����ݷ�����֪����1����Ҫ�ɷ�ΪPt��Au��
3Cu2����2NO����4H2O�����ݷ�����֪����1����Ҫ�ɷ�ΪPt��Au��
(2)�ڢڲ������м�H2O2��Ŀ���ǽ�Fe2��ת��ΪFe3�����Ӷ���Fe3��ת��Ϊ������ȥ����ֹ��CuSO4������Ʊ��������š�H2O2���ŵ��ǻ�ԭ����ΪH2O�������������ʣ�ͬʱ�Ի���û����Ⱦ������pH��Ŀ���ǽ�Fe3����Al3��ת��Ϊ��������ȥ��
(3)��CuSO4·5H2O�Ʊ���ˮCuSO4��ֻҪ��ȥ�ᾧˮ���ɡ��ڼ��ȹ�����CuSO4����ˮ�⣬������Ϊ����ӷ����������յõ�����Ȼ��CuSO4������ֻҪ������ˮ���ɡ�
(4)�����Ʊ���Al2(SO4)3�����к���Fe2(SO4)3���ҡ������ַ����У��ҷ�����ԭ�������ʸ��ߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У������չ�������ʱ�����õ���������________________________
(������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о�С��ͨ��ʵ��̽��Cu���仯��������ʣ�������ȷ���ܴﵽʵ��Ŀ�ĵ���(����)
A����ͭ˿����Ũ�����в����ȣ��ѷ�Ӧ�����Һ����ˮ�У��۲�����ͭ��Һ����ɫ
B�������½�ͭ˿����ʢ�������ļ���ƿ�У��۲�CuCl2������
C����CuCl2��Һ���������м������ɣ��õ���ˮCuCl2����
D����������ͭ��[Cu2(OH)2CO3]��ͭ�����������н��ݣ���ȥͭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�
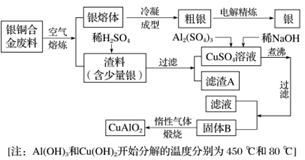
(1)��⾫����ʱ��������ӦʽΪ___________________________________________________��
����A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ��Ӧ����ʽΪ____________��
(2)��������B�����Ϊ__________�������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ____________��
(3)������չ�����һ����Ӧ�Ļ�ѧ����ʽ��____CuO��____Al2O3 ____CuAlO2��________����
____CuAlO2��________����
(4)����ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0 kg�����е�ͭ����ȫת��Ϊ________ mol CuAlO2��������Ҫ 1.0 mol·L��1��Al2(SO4)3��Һ________ L��
(5)CuSO4��ҺҲ�������Ʊ������������������________�����ˡ�ϴ�Ӻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧС��Ϊ�ⶨһ��������ijͭ���������ͭ���������������������ʵ�鷽����
������ͭ������� �ⶨ������������
�ⶨ������������
������ͭ�������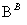 �ⶨʣ����������
�ⶨʣ����������
�����й��ж��в���ȷ����(����)
A����ҺA��B�������������NaOH��Һ
B����ҺA��B������ѡ��ϡ����
C������ҺBѡ��Ũ���ᣬ����ͭ����������ƫС
D��ʵ�鷽���������ʵʩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ṩ���Լ��ͷ�����ȥ����ĩ״������е�����(������Ϊ����)������ѡ�𰸵ı�������±�����Ӧ�Ŀո���(�������Ҫ����Լ������Ӧ�����ɿ���)��
�ɹ�ѡ����Լ���A.���ᡡB������������Һ��C������
D��ˮ��E��������̼
��ѡ�õIJ�������ˮϴ���ڼ��ȡ��۸������ա��ܹ���
�ݽᾧ
| ��ĩ״����� | ѡ����Լ� | ѡ�õIJ��� | |
| (1) | CaCO3(SiO2) | ||
| (2) | NaCl(SiO2) | ||
| (3) | SiO2(Fe2O3) | ||
| (4) | SiO2(CaCO3) | ||
| (5) | SiO2(NH4Cl) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯ����һ���°����ʣ��ǡ�¹�ص���Լ���������Ƶ�46�ֻ�ѧƷ֮һ���仯ѧʽΪNa2Fe5Si8O22(OH)2����ʯ����ϡ������Һ����ʱ����ԭ����ֻ��NO������˵������ȷ����(����)
A����ʯ����һ�ֹ����β���
B����ʯ���к���һ������ʯӢ����
C����ʯ�Ļ�ѧ��ɿɱ�ʾΪNa2O·3FeO·Fe2O3·8SiO2·H2O
D��1 mol��ʯ����ʹ1 mol HNO3����ԭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������CO2ͨ��KOH��Ca(OH)2�Ļ��ϡ��Һ�У����ɳ��������ʵ���(n)��ͨ��CO2���(V)�Ĺ�ϵ��ȷ����(����)
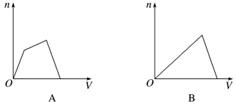
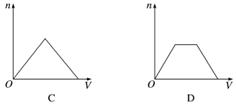
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)���ȹ������Է����е������ԣ�������һ�����Է����У����ȹ���û���Է����е������ԣ�����һ��������Ҳ���Է�����(����)
(2)��Ӧ�ܷ��Է��������ۺϿ����ʱ���ر�Է�Ӧ��Ӱ��(����)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com