
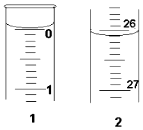
 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�| �ζ� ���� | �������������� Һ�����/mL | 0.1000mol•L+1����������mL�� | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 |
| ������ | 25.00 | 0.22 | 26.31 |
���� ��1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯��
��2������c�����⣩=$\frac{c��������V������}{V�����⣩}$����������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��3������ͼʾ��ȡ�ζ�ǰ��̶�ֵ��
��4�����������NaOH��Ӧ���C��NaOH�ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ�����Ÿ��������NaOH��Ӧ���C��NaOH����
��� �⣺��1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ƿ����Һ��ɫ�ı仯��
��2��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨc��NaOH��ƫ��A�����ϣ�
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨc��NaOH����Ӱ�죬��B�����ϣ�
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨc��NaOH��ƫ��C�����ϣ�
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨc��NaOH��ƫ�ͣ���D���ϣ�
�ʴ�Ϊ��D��
��3����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��
�ʴ�Ϊ��26.10��
��4���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=26.10mL��
HCl+NaOH=NaCl+H2O
0.02610L��0.1000mol/L 0.025L��C��NaOH��
��C��NaOH��=$\frac{0.02610L��0.1000mol/L}{0.025L}$=0.1044mol/L��
�ʴ�Ϊ��0.1044mol/L��
���� ������Ҫ�������к͵ζ��������������Լ����㣬�ѶȲ��������к͵ζ���ԭ���ǽ���ؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬ������H2��Cl2���еķ�������� | |
| B�� | SO42-��Ħ��������96 g•mol-1 | |
| C�� | �����ӵ�����ǡ��Ϊ6.02��1023mol-1 | |
| D�� | 1 mol CO2������Ϊ44 g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| A | B | C | D | E | F | G | H | I | J | |
| ԭ�Ӱ뾶��10-10 m�� | 1.02 | 2.27 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.86 | 0.75 | 1.17 |
| ���̬ | +6 | +1 | - | +3 | +4 | +5 | +7 | +1 | +5 | +4 |
| ��ͼ�̬ | -2 | - | -2 | - | -4 | -3 | -1 | - | -3 | -4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 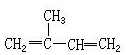 2-��-1��3-����ϩ 2-��-1��3-����ϩ | B�� | 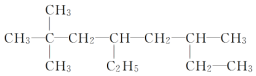 2��2-����-4��6-���һ����� 2��2-����-4��6-���һ����� | ||
| C�� |  1��3��4-���ױ� 1��3��4-���ױ� | D�� |  2-��-3-��ϩ 2-��-3-��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
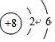 ����Ԫ��ͨ��ת��Ϊ������Σ�笠����ӵĵ���ʽΪ
����Ԫ��ͨ��ת��Ϊ������Σ�笠����ӵĵ���ʽΪ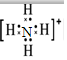 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | SO2��Cl2��������Ư�����ʣ��������ʵ�������������ͬʱ�����ڳ�ʪ����ɫ����ʱ��������ǿƯ��Ч�� | |
| B�� | SO2��ʹƷ����Һ����ˮ�����Ը��������Һ��ɫ������ΪSO2����Ư���� | |
| C�� | SO2ͨ����ɫʯ����Һ�У���Һֻ��첻��ɫ | |
| D�� | Cl2ͨ����ɫʯ����Һ�У���Һ�ȱ�����ɫ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com