
����Ŀ���õ������(��Ҫ��![]() ��
��![]() )���������Ȼ������������������壬�乤���������£�
)���������Ȼ������������������壬�乤���������£�

��֪����![]() ��ˮ�����ɼ�ʽ�Ȼ�����
��ˮ�����ɼ�ʽ�Ȼ�����![]() ������ˮ��Һ�У�
������ˮ��Һ�У�![]() �ױ�����Ϊ
�ױ�����Ϊ![]() ��
��
��ش��������⣺
(1)�������ڱ��е�λ��Ϊ___________��
(2)��֪��ӦI�õ��ij�����![]() ����������ĽṹʽΪ_____����Ӧ�Ļ�ѧ����ʽΪ_______��
����������ĽṹʽΪ_____����Ӧ�Ļ�ѧ����ʽΪ_______��
(3)ͼ����Һ����Ҫ�ɷ�Ϊ_______________��_______________(д��ѧʽ)��
(4)����ʱһ�����Ũ��������ܽ⣬���û�ѧ����ʽ����Ҫ���ֽ���ԭ��______________��
(5)��������е������ռ��������Һ��Ӧ�����������ƣ�������һ�ּ������壬�÷�Ӧ�Ļ�ѧ����ʽΪ________��
���𰸡��������ڵ���A�� O��C��O Na2CO3+SnCl2 �� SnO��+2NaCl+CO2�� NaCl Na2CO3 SnCl2����ˮ�⣬����ƽ��SnCl2+H2O![]() Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�� 2Sn+3NaOH+NaNO3 �� 2Na2SnO3+NH3��
Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�� 2Sn+3NaOH+NaNO3 �� 2Na2SnO3+NH3��
��������
��������е�Sn��SnO�����ᷴӦ����SnCl2��ϴ�Ӳ��ᾧ��õ�SnCl2���壬���þ����ܽ��������з�ֹSnCl2ˮ�⣬���������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ�������м���Sn�ۣ�Sn�ۿ��Ժ�H+������Ӧ��ʹ��Һ���Լ�������������ҺpH������Sn���Խ����������ɵ�Sn4+��ԭ��Sn2+������ֹSn2+������ΪSn4+�����˵�SnCl2��Һ�������м�̼���ƣ���SnԪ����SnO��ʽ������������Ӧ�ķ���ʽΪNa2CO3+SnCl2 �� SnO��+2NaCl+CO2��������ϴ�ӵô�����SnO����Ӧ����Һ������ΪNaCl��Na2CO3��SnO�м�ϡH2SO4����SnSO4��Һ����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵ�ϵ�в������Ƶ�SnSO4���塣
(1)��Ԫ����̼Ԫ������ͬһ���壬������A�壬ԭ�Ӻ˵����Ϊ50����50281818��4����Sn���ڵ������ڣ��������ڱ��е�λ��Ϊ���������ڵ���A�壻
(2)��Ӧ���Ļ�ѧ����ʽΪNa2CO3+SnCl2 �� SnO��+2NaCl+CO2�������ɵ�����ΪCO2��CO2Ϊ���ۻ������ṹʽΪO��C��O��
(3)���Ϸ�����Һ������ΪNaCl��Na2CO3��
(4)����Ϣ��֪��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����������ƽ��SnCl2+H2O![]() Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻
Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻
(5)��Ӧ��ΪSn��NaOH��NaNO3��������ΪNa2SnO3��NH3������Sn+NaOH+NaNO3��Na2SnO3+NH3�������ݵ�ʧ�����غ��Ԫ���غ㣬�ɵ�2Sn+3NaOH+NaNO3 �� 2Na2SnO3+NH3����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п�ڵ�����췽��������Ҫ�����ã�Ҳ������������Ԫ��֮һ�����볣���ķǽ����������γ���Ҫ�Ļ����
��1��Zn�ĺ�������Ų�ʽ��[Ar]___��
��2��Zn�ĸ����������������±���ʾ��

�������ݱ�������˵��п�ij������ϼ�Ϊ��2��ԭ����___��
�������������ֳ����ǽ����ĵ縺�ԣ��ɴ�С��˳����___�����Ȼ���Ϊ�ʺ�ɫҺ�壬�۵�-78�棬���侧���������������Ӧ����___�����ķ���������ԭ�Ӿ��еŵ��Ӷ�����___��
��3���Ȼ�п������ˮ����ˮ���γ������H[ZnCl2(OH)]��H[ZnCl2(OH)]��ˮ�е���ʱ�����ӷ���ʽΪ___��
��4�������Ŀ�϶�����Ǿ����о�����Ҫ���ݡ�
����֪���������������������϶�Ͱ������϶״����ͼ��ʾ����������������ÿ�������У��ѻ��������������϶�����������϶��=___��
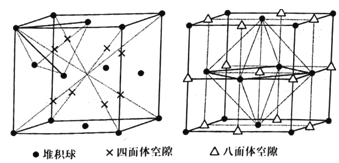
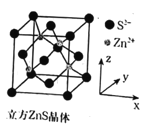
�ڸ�������ZnS����ʾ��ͼ��������ÿ�������У�п��������������ӵ��������ľ�����϶�еķ�ʽΪ___�����������Ϊ(0��0��0)�������������п�����������Ϊ___ (������ֵ��Ϊ��ֵ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о����̽����㺬�е�Ag(�������������Բ���)����������е�Ag2O�ȷϾ���Դ�Ļ������������ش���ͼΪ�ӹ�������ȡAg�Ĺ�ҵ���̣���ش��������⡣
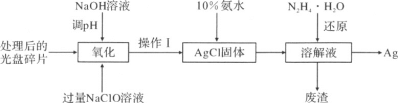
(1)NaClO��Һ�����Ȼ������������ֽ⣬��������������80�������½��У����˵ļ��ȷ�ʽΪ _____��
(2)NaClO��Һ��Ag��Ӧ�IJ���ΪAgCl��NaOH��O2���÷�Ӧ�Ļ�ѧ����ʽΪ______�����������HNO3����NaClO����Ag���ӷ�Ӧ����ĽǶȷ�������ȱ����_______��
(3)�����������Ϊ_______����ѧ�ϳ���10���İ�ˮ�ܽ�AgCl���壬AgCl��NH3��H2O��1��2��Ӧ������Cl����һ��������__________����Һ(�������ӵĻ�ѧʽ)��ʵ�ʷ�Ӧ�У���ʹ��ˮ����Ҳ���ܽ�AgCl����ȫ���ܽ⣬���ܵ�ԭ����_______��
(4)����ʱN2H4��H2O(ˮ����)�ڼ����������ܻ�ԭ(3)�����ɵ������ӣ�����ת��Ϊ������N2������������0.1 mol��ˮ���¿���ȡ�� ______g�ĵ���Ag��
(5)�Ͼɵ����Ag2O�ܽ��ж������ȩ(HCHO)������CO2����ѧ�Ҿݴ�ԭ���������������Ϊԭ��ػ��յ缫����Ag����Чȥ��������ȩ����˵�ص�������ӦʽΪ _____�������IJ�����____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+���� ��Na+ˮ ��ش��������⣺

��1��д���١��ڷ�Ӧ�����ӷ���ʽ
��__________________________________________��
��________________________________________________________
��2���ڵ�ȼH2֮ǰ�����Ƚ���_______________________________________��
��3��ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ����ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ��___________________________________________________________________
��4��ʵ��С������ơ�����ˮ���ܶȷֱ�Ϊ0.97g/mL��0.88g/mL��1.00g/mL�����ݴ˶�ʵ������˸Ľ����ڸĽ����ʵ����H2���������ʻ�_______________���Ľ����ʵ������Ϊ_________________________________________________________________________��
��5��2.3 g��Ͷ��20 mLˮ����ȫ��Ӧ�ų��������ڱ�״���µ������____________,������Һ�����ʵ���Ũ����______________________��(������Һ����ı仯)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������;��㷺�Ľ������ϡ�
��1�������ۺͻ���̿�Ļ������NaCl��Һʪ���������ͼ��ʾװ���У��������ĵ绯ѧ��ʴʵ�顣д��������Ӧʽ��______��

��2���������缫����Ũ��NaOH��Һ�����Ƶ�Na2FeO4��װ����ͼ��ʾ�������ĵ缫��ӦʽΪ______��������11.2g������ͨ�����ӽ���Ĥ��Na�����ʵ���Ϊ______��

��3����ͼ��ʾװ��Ϊ���ü���ȼ�ϵ��ʵ�������ʲ����϶�п��һ���֣�ȼ�ϵ�صĸ�����ӦʽΪ______������ͼ��ʾ���߿��ڲ������������ʲ����϶�п��װ��ͼ(ע���缫���Ϻ͵������Һ�ijɷ�)��_____
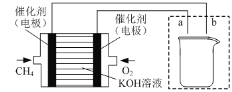
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ����������ƽ�������������и�����һֻ�ձ���������ƽʹ֮ƽ�⣬���ձ��зֱ�ע�������ҵ���������Ũ�ȵ�ϡ���ᣬȻ������ֻ�ձ��зֱ������ͬ������пƬ�������Ͻ𣬷�Ӧ��ɺ���ƽ�Ա���ƽ�⡣�Ͻ�����������������Ϊ(����)
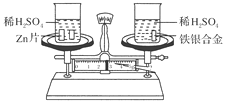
A.65 ��56B.56 ��108
C.56 ��9D.65 ��9
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���ѧ��Ӧ�е������仯�����ڸ��õĿ�����ʹ�û�ѧ��Դ��
��1������þ[Mg(s)]�ֱ���±�ص���[F2(g)��Cl2(g)��Br2(l)��I2(s)]��Ӧ�������仯��ͼ��ʾ��

��д��Mg(s)��I2(s)��Ӧ���Ȼ�ѧ����ʽ��_______��
����Ӧ��MgCl2(s)��F2 (g)��MgF2(s)��Cl2 (g)����H��___________��
��2��CH4��NiO��������Ļ�ѧ��ȼ��ʾ��ͼ��ͼ��ʾ��

��Ҫ�Ȼ�ѧ��Ӧ���£�2Ni(s)��O2(g)��2NiO(s) ��H����479.8 kJ��mol��1��CH4(g)��4NiO(s)��CO2(g)��2H2O(l)��4Ni(s) ��H����68.9 kJ��mol��1
��CH4��ȼ������_______��
��CH4������ѧ��ȼ���������ڶ�����̼�ķ�������գ����ų�����������ͬ��������CH4��ֱ��ȼ�����_________������ǰ�ߴ����������ߴ�����������ͬ������
��3����֪H2��CO��ȼ���ȷֱ�Ϊ285.8 kJ��mol��1��283.0 kJ��mol��1��25��ʱ��4 g H2��28 g CO�Ļ��������ȼ�գ��ָ���ԭ�£��ܷų�������Ϊ_______kJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ե������Ǵӷ仨��ֲ������ȡ�õ����������ʣ���ṹ��ʽ��ͼ��ʾ������������ȷ���� (����)

A. �Ե��������嵥��ֻ�ܷ���ȡ����Ӧ
B. 1 mol�Ե���������ܺ�9 mol���������ӳɷ�Ӧ
C. �Ե�������Է���ˮ�ⷴӦ��ȡ����Ӧ����ȥ��Ӧ��������Ӧ
D. 1 mol�Ե���������ܺ�6 mol NaOH������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪3.25g����Znǡ����250mLijŨ�ȵ�������ȫ��Ӧ�����㣺
��1������������ʵ���Ũ�ȣ�___________
��2����״���£��÷�Ӧ���ɵ�����������_____________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com