
����Ŀ��NH3��N2H4�ڹ�ҵ�������������ж��й㷺Ӧ�á��ش��������⣺
��1����N2H4 (g) ![]() N2(g)+2H2(g) ��H1
N2(g)+2H2(g) ��H1
��N2(g)+3H2(g) ![]() 2NH3(g) ��H2
2NH3(g) ��H2
��7N2H4(g) ![]() 8NH3(g)+3N2(g)+2H2(g) ��H3
8NH3(g)+3N2(g)+2H2(g) ��H3
��H3=___________���ú���H1�͡�H2�Ĵ���ʽ��ʾ����
��2�������ܵĴ������£�N2H4�ɷֽ������������壬����һ����ʹʪ��ĺ�ɫʯ����ֽ����������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ����������ͼ��ʾ��

�÷�Ӧ�ġ�H________���>����<����0��N2H4�����ֽⷴӦ�Ļ�ѧ����ʽΪ___________��
��3��T��ʱ����һ���Ϊ5L �ĺ����ܱ������г��������ʵ���Ϊ2 mol��CO2��NH3����һ�������·�����Ӧ��2NH3(g)+CO2(g��====CO(NH2)2(s)+H2O(g)����������а�������������淴Ӧʱ��ı仯��ͼ��ʾ��

�� 0��60s�ڣ���Ӧ����v(CO2)=________mol/(L��s)
�� T��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=________��
���𰸡� 7��H1+4��H2 > 3N2H4![]() 4NH3+N2 1.25��10-3 240
4NH3+N2 1.25��10-3 240
����������1����֪��
��N2H4(g)![]() N2(g)+2H2(g) ��H1
N2(g)+2H2(g) ��H1
��N2(g)+3H2(g)![]() 2NH3(g) ��H2
2NH3(g) ��H2
��7N2H4(g)![]() 8NH3(g)+3N2(g)+2H2(g) ��H3
8NH3(g)+3N2(g)+2H2(g) ��H3
����ݸ�˹���ɿ�֪�١�7+�ڡ�4���õ���Ӧ�۵���H3=7��H1+4��H2��
��2������һ����ʹʪ��ĺ�ɫʯ����ֽ�������������ǰ���������ͼ���֪���¶����ߣ�����������������ӣ���˵�������¶�ƽ��������Ӧ������У���÷�Ӧ����H��0������ͼ���֪��������������֮�Ƚ���Ϊ4:1�����Ը���ԭ���غ��֪N2H4�����ֽⷴӦ�Ļ�ѧ����ʽΪ3N2H4![]() 4NH3+N2��
4NH3+N2��
��3���ٸ���ͼ���֪��ʼʱ���ߵ������ȣ���˾���1mol��Ũ�Ⱦ���0.2mol/L��ƽ��ʱ�������������0.2������ݷ���ʽ��֪
2NH3(g)+CO2(g��![]() CO(NH2)2(s)+H2O(g)
CO(NH2)2(s)+H2O(g)
��ʼŨ�ȣ�mol/L�� 0.2 0.2 0
ת��Ũ�ȣ�mol/L�� 2x x x
ƽ��Ũ�ȣ�mol/L�� 0.2-2x 0.2-x x
��![]() �����x��0.075
�����x��0.075
��0��60s�ڣ���Ӧ����v(CO2)=0.075mol/L��60s��1.25��10-3mol/(L��s)
�� T��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��W��X��Y��Z��ԭ�������������ӡ�m��n��p������ЩԪ����ɵĶ�Ԫ�����W2��X2��Z2�ֱ���Ԫ��W��X��Z�ĵ��ʡ���֪I.һ��������ij�ܱ������пɷ�����ӦaX2+bW2![]() cm����Ӧ���������ʵ�Ũ�ȱ仯���£�
cm����Ӧ���������ʵ�Ũ�ȱ仯���£�
X2 | W2 | m | |
��ʼŨ��/mol/L | 0.4 | 0.4 | 0 |
ƽ��Ũ��/mol/L | 0.3 | 0.1 | 0.2 |
�����ǿɷ������·�Ӧ��2m(g)+3Z2(g)=6n(g)��X2(g)��4n(g)+Y2(g)![]() 2p(l)+2Z2(g)������˵����ȷ����
2p(l)+2Z2(g)������˵����ȷ����
A. m��n��p�������ʾ�Ϊ���ۻ�����
B. a:b:c=3:1:2
C. X��������һ������ɫ����
D. ԭ�Ӱ뾶��W<X<Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д���з�Ӧ�����ӷ���ʽ��
��1��Na2CO3��Һ������ķ�Ӧ��___________________��
��2��Ba(OH)2��Һ��CuSO4�ķ�Ӧ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л��������˵����ȷ����
A. �Ҵ���ˮ��Һ�׳ƾƾ�
B. �ɱ���������һ������������C6H6Cl6�ķ�Ӧ����ȡ����Ӧ
C. ��ѧʽΪC4H10O�ı���һԪ����4��
D. �����ˮ�ⷴӦ�����ղ��ﶼ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�����缫һΪ̼����һΪ��Ƭ������������ָ�뷢��ƫת����a�����д����������ɣ�������������ȷ����

A. aΪ����������Ƭ���ձ��е���ҺΪ����
B. bΪ����������Ƭ���ձ��е���ҺΪ����ͭ��Һ
C. aΪ��������̼�����ձ��е���ҺΪ����
D. bΪ��������̼�����ձ��е���ҺΪ����ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�������ȷ���ǣ� ��
A.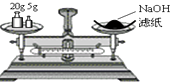 �����������ƹ���
�����������ƹ���
B. ������ͺ�ˮ
������ͺ�ˮ
C. �ѱ���ʳ��ˮ�е�ʳ����ȡ����
�ѱ���ʳ��ˮ�е�ʳ����ȡ����
D. �������ֻ��ܵ��е����ϴ��Һ������
�������ֻ��ܵ��е����ϴ��Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����λ�����Ⱦ��������̬�����ѳ�Ϊȫ��Ĺ�ʶ��
�ٿ�����������ĸ���ָ�������ֿ��������������и���ָ�겻��Ҫ������___________��
A������������PM2.5�� B��NO2Ũ�� C��SO2Ũ�� D��CO2Ũ��
�����д�ʩ�����ڸ��ƻ�����������___________��
A�����ϵ�������Լ����ؽ�������Ⱦ
B�����ع��ͻ����ټӹ�Ϊʳ���ͣ��Լ���ˮ�帻Ӫ����
C�����ö�����̼��ԭ�Ϻϳɾ�̼������ɽ������ϴ������ϩ
��2����ȡ�ʵ��Ĵ�ʩ�ܼ��ٶԻ�������Ⱦ��
��������ָpH___________�Ľ�ˮ������ȼ�պ���ú������������ˮ���䵽���棬pH��ʱ��䳤��������С�����û�ѧ����ʽ������ԭ��___________��
��һ����̼Ҳ�dz����Ĵ�����Ⱦ������������������еIJ���ȫȼ�տɱ�ʾΪ2C8H18+23O2![]() 12CO2+4CO+18H2O
12CO2+4CO+18H2O
ij���������ȤС���ͬѧ���������ĵ���������CO�����仯����(�������ʾ����ʱ��0��24Сʱ���������ʾCO����)������Ϊ�ȽϷ���ʵ�ʵ���____________��

A B C D
����Ȼˮ�к��е�ϸС��������������___________����������д���ƣ����о�����������ҵ��ˮ�к��е�Cr3+���ӣ�������ʯ��������������pHΪ8��9ʱ���ɳ�������ȥ���÷�Ӧ�����ӷ���ʽ��_____________��
��3��ʳƷ��ҩƷ��ϵ�˵�����ͽ�����
���г������۵�ʳ��Ʒ�ֺܶࡣ����ʳ���У������ӵ�Ԫ�ز��������������Ԫ�ص���___________������ĸ����
A����п�� B���ӵ��� C���Ӹ���
����ά�ر���Ϊ������Ӫ��������ʳ���е���ά����Ȼ����Ϊ�����ṩ���������ܴٽ������䶯�������ų��к����ʡ��ӻ�ѧ�ɷֿ�����ά����һ��___________������ĸ����
A������ B�������� C��֬��
��ijͬѧ��ð���գ����ɷ�����������ҩƷ��������____________������ĸ����
A����Ƽ��� �� B����˾ƥ�� ��C������ҩ
��4����ѧ�����������ء�
���˹��ϳɵ������ж��֣����о���ϩ�dz�����������Ʒ���ṹ��ʽ��___________��
�ڸ����ĸ�ʴ��Ҫ����������ʴ���������ĵ缫��ӦΪ___________��Ϊ��ֹ�ִ��Ĵ����ں�ˮ�б���ʴ��һ���ڴ�������_____________������п��������ͭ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���¶�ΪTʱ.��2.0 L�����ܱ������г���1.0 mol PCl5��������ӦPCl5 (g)![]() PCl3(g)+Cl2(g)������һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ�IJ������������±�������˵����ȷ����
PCl3(g)+Cl2(g)������һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ�IJ������������±�������˵����ȷ����
t/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3)/mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
A. ��ǰ50s��PCl3��ƽ����Ӧ���� v(PCl3)=0.0032mol/(L��s)
B. �ﵽƽ��ʱ�������е�ѹǿ����ʼʱ��1.2��
C. ��ͬ�¶�������ʼʱ�������г���1.0mo PCl5��0.20 mo1 PCl3��0.20 mo1 Cl2����Ӧ�ﵽƽ��ǰv(��)>v(��)
D. ��ͬ�¶�������ʼʱ�������г���2.0 mol PCl3��2.0 mol Cl2���ﵽƽ��ʱ��PCl3��ת����С��80%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ�����
��1��1.5mol HNO3Լ��������ԭ�ӣ���0.6mol H ��C6H12O6�����ʵ�����mol��
��2��22��CO2�����ʵ���Ϊmol���������ڱ�״���µ����ΪL��
��3��4gNaOH��������ʵ���Ϊmol����������ˮ���500mL��Һ������Һ��NaOH�����ʵ���Ũ��Ϊ ��
��4����5mol/L����10mLϡ�͵�200mL��ϡ�ͺ���Һ�����ʵ���Ũ���� ��
��5��ʵ�����Ʊ�Fe ��OH��3����Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com