
��1��2012���ذ��˻�����ñ���Ϊȼ�ϡ�������ֵ�ϸߣ���Ⱦ��С����һ��������ȼ�ϡ��Իش��������⣺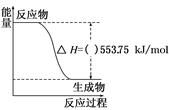
����ͼ��һ����������ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ������ͼ�е����������롰����������
��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��___________________________________��
�۶�����(CH3OCH3)��һ������ȼ�ϣ�Ӧ��ǰ��������1 mol��������ȫȼ������CO2��Һ̬ˮ�ų�1 455 kJ��������1 mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1 645 kJ���������������У�����Ͷ����ѵ����ʵ���֮��Ϊ________��
��2����˹������Ϊ�����ܻ�ѧ������һ����ɻ�ּ�����ɣ��������̵�����ЧӦ��ͬ�������ø�˹���ɻش��������⣺
����֪��H2O(g)=H2O(l)����H1����Q1 kJ/mol C2H5OH(g)=C2H5OH(l)����H����Q2 kJ/mol
C2H5OH(g)��3O2(g)=2CO2(g)��3H2O(g)����H3����Q3 kJ/mol
��ʹ46 gҺ̬��ˮ�ƾ���ȫȼ�գ����ָ������£������������зų�������Ϊ________kJ��
��̼(s)��������Ӧ������ʱ������COͬʱ����������CO2�������ͨ��ʵ��ֱ�Ӳ�÷�Ӧ�� C(s)��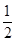 O2(g)=CO(g)�Ħ�H���������ʵ�顢���ø�˹���ɼ�����÷�Ӧ�Ħ�H������ʱ��Ҫ��õ�ʵ��������________��
O2(g)=CO(g)�Ħ�H���������ʵ�顢���ø�˹���ɼ�����÷�Ӧ�Ħ�H������ʱ��Ҫ��õ�ʵ��������________��
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)��H2O(g)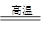 CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C(s)��O2(g)=CO2(g)����H����393.5 kJ��mol��1
H2(g)�� O2(g)=H2O(g)����H����242.0 kJ��mol��1
O2(g)=H2O(g)����H����242.0 kJ��mol��1
CO(g)�� O2(g)=CO2(g)����H����283.0 kJ��mol��1
O2(g)=CO2(g)����H����283.0 kJ��mol��1
��ش�
(1)�����������ݣ�д��C(s)��ˮ������Ӧ���Ȼ�ѧ����ʽ��________��
(2)�ȽϷ�Ӧ�����ݿ�֪��1 mol CO(g)��1 mol H2(g)��ȫȼ�շų�������֮�ͱ�1 mol C(s)��ȫȼ�շų��������ࡣ��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹������������ѭ��ͼ��
���ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
��������ס�����ͬѧ�۵���ȷ����________(��ס����ҡ�)���жϵ�������________��
(3)��úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ�________��
(4)ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϡ�CO��H2��һ�������¿��Ժϳɣ��ټ״����ڼ�ȩ���ۼ�������ᡣ�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ�����________(�����)����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷš�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��ѧ��ӦN2��3H2 2NH3�������仯��ͼ��ʾ��
2NH3�������仯��ͼ��ʾ��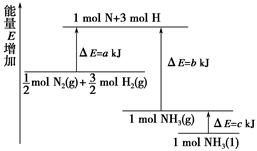
(1)1 mol N��3 mol H����1 mol NH3(g)��________�����Ĺ���(����ա����ͷš�)��
(2)�� mol N2(g)��
mol N2(g)��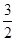 mol H2(g)����1 mol NH3(g)����________(����ա����ͷš�)________kJ������
mol H2(g)����1 mol NH3(g)����________(����ա����ͷš�)________kJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���������Ҫ��ӦΪ��4NH3��g��+5O2��g��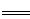 4NO��g��+6 H2O��g����H��
4NO��g��+6 H2O��g����H��
��1����֪������ȼ����Ϊ285��8 kJ/mol��
N2��g��+3H2��g��=2NH3��g�� ��H=��92��4 kJ/mol;
H2O��1��=H2O��g����H=+44��0 kJ/mol;
N2��g��+O2��g��=2NO��g����H=+180��6 kJ/mol��
��������ҵ���������Ҫ��Ӧ�ġ�H= ��
��2�����ݻ��̶����ܱ������з���������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���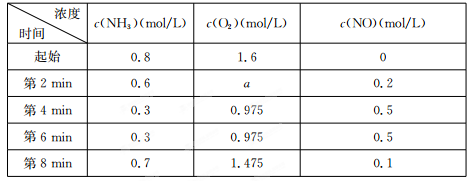
�ٷ�Ӧ�ڵ�2 min����4 minʱ��O2��ƽ����Ӧ����Ϊ ��
�ڷ�Ӧ�ڵ�6 minʱ�ı����������ı������������ ������ţ���
A��ʹ�ô��� B�������¶� C����Сѹǿ D������O2��Ũ��
������˵������˵��4NH3��g��+5O2��g�� 4NO��g��+6 H2 O��g���ﵽƽ��״̬���� ������ţ���
4NO��g��+6 H2 O��g���ﵽƽ��״̬���� ������ţ���
A����λʱ��������n mol NO����ʱ������n mol NH3
B������һ������������ƽ����Է����������ٱ仯
C���ٷֺ���w��NH3��=w��NO��
D����Ӧ����v��NH3����u��O2����v��NO����v��H2O��=4��5��4��6
E�����ں��º�ѹ���ݻ��ɱ�������з�Ӧ�����������ܶȲ��ٱ仯
��3��ij�о�����װ��CH3OH��O2ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��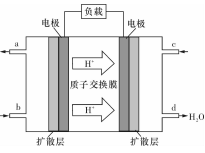
�ٸõ�ع���ʱ��b��ͨ�������Ϊ____ ��
�ڸõ�������ĵ缫��ӦʽΪ�� ��
���Դ˵������Դ����ʵ������ģ������Ʒ���桰�ۻ���������װ����ͼ��ʾ���Ĺ����У�������Һ����Dz������ݲ�������ԭ������� ������ص����ӷ���ʽ��ʾ����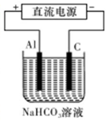
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�������Դ�����ʣ����ٻ�����Ⱦ���������Ž��ѳ����ȼ�ͼ״�����ɲ�ҵ������ͼ��ʾ��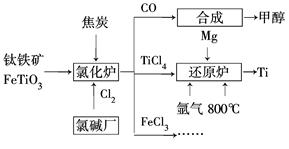
����д���пհס�
(1)����������Ȼ�¯ǰͨ����ȡϴ�ӡ����顢��ɡ�Ԥ�ȵ������������������ԭ���Ͻ��ͷ�������ã�_______________________________________
��֪�Ȼ�¯�������ͽ�̿�������������ʵ���֮��Ϊ7��6�����Ȼ�¯�л�ԭ���Ļ�ѧʽ��___________________________��
(2)��֪����Mg(s)��Cl2(g)=MgCl2(s)��H����641 kJ/mol
��2Mg(s)��TiCl4(s)= 2MgCl(s)��Ti(s)��H����512 kJ/mol
��Ti(s)��2Cl2(g)=TiCl4(s)����H��________��
(3)���ͨ�뻹ԭ¯�в������뷴Ӧ��ͨ�������������___________________________
(4)�Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ2CH3OH��3O2��4OH��=2CO32����6H2O���õ���������ϵĵ缫��ӦʽΪ_________________________________________��
����һ��ʱ������Һ��pH________(���С�����������䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ŀǰ���緶Χ�ڵ���ԴΣ�����״���Ϊһ�ֽϺõĿ�������Դ�����й㷺��Ӧ��ǰ����
��1����֪�ڳ��³�ѹ�·�Ӧ���Ȼ�ѧ����ʽ��
��CO��g����2H2��g��  CH3OH��g������H1����90 kJ��mol��1
CH3OH��g������H1����90 kJ��mol��1
��CO��g����H2O��g��  CO2��g����H2��g����H2����41 kJ��mol��1
CO2��g����H2��g����H2����41 kJ��mol��1
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��_______________________��
��2�����ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ʱ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
��p1________p2������ڡ�����С�ڡ����ڡ�����
���������������������£�������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת����________�����������С�����䡱����ͬ����ƽ�ⳣ��________��
��3����֪��T ��ʱ��CO��g����H2O��g��??CO2��g����H2��g����ƽ�ⳣ��K��0.32���ڸ��¶��£���֪cʼ��CO����1 mol��L��1��cʼ��H2O����1 mol��L��1��ijʱ�̾��ⶨCO��ת����Ϊ10%����÷�Ӧ________����Ѿ�����û�С����ﵽƽ�⣬ԭ����___________________________________________����ʱ��v��________v�����>����<������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.���û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч������������������ϩ��Ϊ��ԭ�������������ﻹԭΪN2��ȼú������һ������(��NOx)������������������ͼ��ʾ��д����������������ϩ��NO2��Ӧ�Ļ�ѧ����ʽ ��
��.(1)��ͼ��1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�����е������仯ʾ��ͼ,���ڷ�Ӧ��ϵ�м������,��Ӧ��������,E1�ı仯�� (���������С�����䡱,��ͬ),��H�ı仯�� ��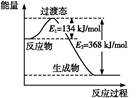
(2)�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���Ǣ�CH3OH(g)+H2O(g)=CO2(g)+3H2(g)��H="+49.0" kJ��mol-1;
��CH3OH(g)+ O2(g)=CO2(g)+2H2(g)��H="-192.9" kJ��mol-1��
O2(g)=CO2(g)+2H2(g)��H="-192.9" kJ��mol-1��
��֪��H2O(g)=H2O(l) ��H="-44" kJ��mol-1��
��״�������ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽΪ ��
д���״����ӽ���Ĥȼ�ϵ�������������µĸ�����Ӧʽ: ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�ڱ���¯�з�����Ӧ��
��Fe2O3(s)��3C(s) 2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
��3CO(g)��Fe2O3(s) 2Fe(s)��3CO2(g)����H����25.2 kJ��mol��1
2Fe(s)��3CO2(g)����H����25.2 kJ��mol��1
��Ӧ2Fe2O3(s)��3C(s) 4Fe(s)��3CO2(g)����H��________kJ��mol��1��
4Fe(s)��3CO2(g)����H��________kJ��mol��1��
(2)��Ȼ��(�Լ����)�ڹ�ҵ��������;�㷺����������ת������H2����Ҫת����Ӧ���£�
CH4 (g)��H2O(g) CO(g)��3H2(g)����H����206.2 kJ��mol��1
CO(g)��3H2(g)����H����206.2 kJ��mol��1
CH4(g)��2H2O(g) CO2(g)��4H2(g)����H����165.0 kJ��mol��1
CO2(g)��4H2(g)����H����165.0 kJ��mol��1
������Ӧ����ԭ�����е�CO��ʹ���ϳɴ����ж��������ȥ����ҵ�ϳ����ô���������CO��ˮ������Ӧ�����׳�ȥ��CO2��ͬʱ�ֿ��Ƶõ�����������ķ������˷�Ӧ��Ϊһ����̼�任��Ӧ���÷�Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��һֱ�����ڡ��˹��̵����ķ����о���
(1)�ϳɰ���ԭ��Ϊ��N2(g)+3H2(g) 2NH3(g)��H="-92.4" kJ/mol���÷�Ӧ�������仯��ͼ��ʾ��
2NH3(g)��H="-92.4" kJ/mol���÷�Ӧ�������仯��ͼ��ʾ��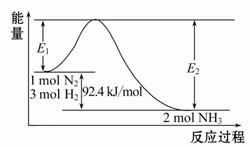
���ڷ�Ӧ��ϵ�м����������Ӧ��������E2�ı仯�� (���������С�����䡱)��
�ڽ�0.3 mol N2��0.5 mol H2�������������ܱ������У���һ�������´ﵽƽ�⣬�������������ѹǿ��Ϊԭ����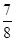 ����ʱH2��ת����Ϊ ������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
����ʱH2��ת����Ϊ ������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
| A���������а�ԭ�����ٳ���ԭ���� |
| B�����������ٳ���һ����H2 |
| C���ı䷴Ӧ�Ĵ��� |
| D��Һ�������������� |
 4NH3(g)+3O2(g)
4NH3(g)+3O2(g) H2O(g) ��H="+44.0" kJ/mol
H2O(g) ��H="+44.0" kJ/mol 4NH3(g)+3O2(g) ��H= kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)��
4NH3(g)+3O2(g) ��H= kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com