
������ͼװ�ã���ɺܶ�绯ѧʵ�飮�����йش�װ�õ������У���ȷ����
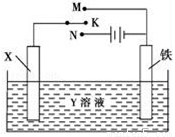
A. ��XΪп����YΪNaCl��Һ������K����M�����ɼ������ĸ�ʴ�����ַ�����Ϊ��������������
B. ��XΪ̼����YΪNaCl��Һ������K����N�����ɼӿ����ĸ�ʴ
C. ��XΪͭ����YΪ����ͭ��Һ������K����M����ͭ�����������ӣ���ʱ���·�еĵ�����ͭ�缫�ƶ�
D. ��XΪͭ����YΪ����ͭ��Һ������K����N�����������������ͭ����Һ��ͭ����Ũ�Ƚ���С
 �ŵ������ϵ�д�
�ŵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ��ѧ�ڵ�һ��ģ�鿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ͬ�������ܱ������зֱ����Ne��H2��O2�������壬�����ǵ��¶����ܶȶ���ͬʱ������������ķ�����(N)�Ӵ�С��˳���ǣ� ��
A. N(Ne)��N(H2)��N(O2) B. N(O2)��N(Ne)��N(H2)
C. N(H2)��N(O2)��N(Ne) D. N(H2)��N(Ne)��N(O2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������и�����Ӧ���¿����壩���ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й���Һ�������������
A. pH��ͬ�Ģ�CH3COONa����NaHCO3����NaAlO2��Һ��c(Na+)���ۣ��ڣ���
B. ��0.2mol��L��1NaHCO3��Һ��0.1mol��L��1KOH��Һ�������ϣ�3c(K+)+c(H+)=c(OH��)+c(HCO3��)+2c(CO32��)
C. �����£���pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ�ĵ���̶���ͬ
D. ��AgCl��AgBr�ĵ����������Һ�м�������AgNO3��Һ����AgCl��������AgBr����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ����3�¸߿���Ӧ�Բ������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ������������أ����������в���ȷ����
A. ������������������Ҫ��Դ
B. ʳ�ο�����ζ����Ҳ����ʳƷ������
C. ��Ȼ���ڵĶ�ֲ����֬��������ڵ�������
D. ��ϩ����ˮ����������ܴٽ�ˮ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ��װ�����ܴﵽʵ��Ŀ����
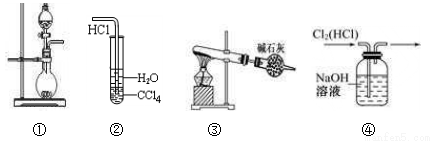
A. ͼ�ٿ�������ȡ����NH3����MnO2��Ũ������ȡCl2
B. װ�âڿ������Ȼ�����������ˮ
C. ��NH4ClΪԭ�ϣ�װ�âۿ�����ʵ�����Ʊ����������NH3
D. װ�âܿ����ڳ�ȥCl2�е�HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
������(H3PO2)��һ�־�ϸ������Ʒ������һԪ���ᣬ���н�ǿ�Ļ�ԭ�ԡ������й�˵����ȷ����
A. �ö��Ե缫���NaH2PO2��Һ����������ӦʽΪ��2H2O-4e-=O2��+4H+
B. H3PO2�����NaOH��Һ��Ӧ�����ӷ���ʽΪ��H3PO2+3OH-=PO23-+3H2O
C. ��H3PO2��Һ���뵽�����ظ������Һ�У�H3PO2�Ļ�ԭ�������ΪH3PO4
D. H3PO2����ˮ�ĵ��뷽��ʽΪH3PO2 H++ H2PO2-
H++ H2PO2-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��Һ�п��ܺ���Fe2����NH4+��Fe3����SO42����Cl���е����ֻ���֡����н�����ȷ����
A. ȡ������Һ�������еμ�AgNO3��Һ�����а�ɫ�������ɣ���˵��һ������Cl��
B. ȡ100 mL��Һ������������NaOH��Һ�����ȣ��ռ�����״����33.6 mL�����壬��c(NH4+)��0.0015 mol��L��1
C. ���ⶨһ�����ĸ���Һ��n(NH4+)��0.02 mol��n(Fe2��)��n(Fe3��)��0.02 mol�����һ��������Һ��0.06 mol��n(Cl��)��0.08 mol
D. ȡ10 mL����Һ��ͨ���״����Cl2 22.4 mLǡ����ȫ��Ӧ��Ȼ�����pH��ʹ��Һ�е���Ԫ��ȫ��ת��Ϊ�����������ˡ�ϴ�ӡ����գ���ȴ����أ��ù�������Ϊ0.32 g������Һ��c(Fe3��)��0.2 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ�ѡ����
���л�ѧ�����ʾ��ȷ����
A. ��ϩ�Ľṹ��ʽ��CH2CH2 B. ����ĵ��뷽��ʽ��H2SO4===2H����SO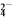
C. S2���Ľṹʾ��ͼ��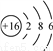 D. CCl4�ĵ���ʽ��
D. CCl4�ĵ���ʽ�� 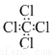
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�ü�����ָʾ��������д���пհף�
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���жϵζ��յ��������__________________��
��2�����в����п���ʹ����NaOH��Һ��Ũ��ƫ�͵���______��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������������Һ�����Ϊ________mL��
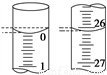
��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
�ζ����� | ����NaOH��Һ�����/mL | 0.100 0 mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.31 | 26.31 |
�ڶ��� | 25.00 | 1.56 | a | 28.74 |
������ | 25.00 | 0.22 | 26.51 | b |
������a����ֵΪ_________�� b����ֵΪ_________��
�����ϱ�������ʽ�����NaOH��Һ�����ʵ���Ũ��Ϊ________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com