

| ÅØĮņĖį |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |
| 46”Į52.17% |
| 12 |
| 46”Į13.04% |
| 1 |
| 46-12”Į2-6 |
| 16 |
| 46”Į52.17% |
| 12 |
| 46”Į13.04% |
| 1 |
| 46-12”Į2-6 |
| 16 |
| ÅØĮņĖį |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |


 ×ŪŗĻ×Ō²āĻµĮŠ“š°ø
×ŪŗĻ×Ō²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ”÷ |
| ”÷ |


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(14·Ö) ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖDŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»īÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©AµÄĆū³ĘŹĒ ”£
£Ø2£©BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
£Ø3£©C+E![]() XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
£Ø4£©Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ (²»ŗ¬A)£ŗ
Ӣ ӣ
£Ø5£©XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
£Ø6£©ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠĪ÷³ĒĒųø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
(14·Ö) ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖDŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»īÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ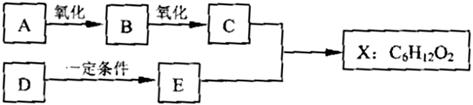
Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©AµÄĆū³ĘŹĒ ”£
£Ø2£©BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
£Ø3£©C+E XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
£Ø4£©Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½(²»ŗ¬A)£ŗ
Ӣ ӣ
£Ø5£©XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
£Ø6£©ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗÓÄĻŹ”ø߶žĻĀµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖ
DŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»ī
ÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā”£
(1)AµÄĆū³ĘŹĒ ”£
(2)BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
(3)C+E XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
(4)Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ (²»ŗ¬A)£ŗ ”£
(5)XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
(6)ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

(1)¹¤ŅµÉś²śÖŠĮ¶ÖĘHµÄ·½·ØŹĒ___________(Ģī”°ČČ·Ö½ā”±”°ČČ»¹Ō”±»ņ”°µē½ā”±)£¬ĘäĮ¶ÖʵĻÆѧ·½³ĢŹ½ĪŖ______________________________________________________________£»
(2)ÉĻŹö±ä»Æ¹ŲĻµÖŠ£¬ŹōÓŚĖ®½ā·“Ó¦µÄŹĒP”śQ¼°__________£¬ŹōÓŚĻūČ„·“Ó¦µÄŹĒ__________£¬ŹōÓŚÖĆ»»·“Ó¦µÄŹĒ__________£»(ÓƱä»Æ¹ŲĻµĶ¼Ź¾±ķŹ¾£¬Čē”°P”śQ”±)
(3)LŅŗĢåÖŠ³ŹŗģŗÖÉ«µÄ·ÖÉ¢ÖŹĮ£×ÓÖ±¾¶ĪŖ__________£»ČēŗĪÓÉKČÜŅŗÖʵĆLŅŗĢå__________£»
(4)ČōRµÄ·Ö×ÓŹ½ĪŖC6H10O4Š“³öÉś³ÉRµÄ»Æѧ·½³ĢŹ½
_____________________________________________________________________£»
(5)±ČMÉŁŅ»øöĢ¼Ō×ÓµÄMµÄĶ¬ĻµĪļX£¬Č”ŗ¬1 mol£ÆL XµÄČÜŅŗ100 mLÓė×ćĮæŠĀÖĘCu(OH)2×ĒŅŗ¼ÓČČ×÷ÓĆ£¬×ī¶ąæÉŅŌµĆµ½×©ŗģÉ«³ĮµķµÄÖŹĮæĪŖ__________g”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com