
NH3(g)Č¼ÉյIJśĪļŹĒNO2(g)ŗĶH2O(g)”£ŅŃÖŖ·“Ó¦ÓŠ£ŗ
(1)H2(g)£«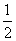 O2(g)===H2O(g) ¦¤H£½£241.8 kJ”¤mol£1
O2(g)===H2O(g) ¦¤H£½£241.8 kJ”¤mol£1
(2)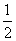 N2(g)£«O2(g)===NO2(g)”” ¦¤H£½£«33.9 kJ”¤mol£1
N2(g)£«O2(g)===NO2(g)”” ¦¤H£½£«33.9 kJ”¤mol£1
(3)NH3(g)===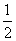 N2(g)£«
N2(g)£«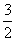 H2(g) ¦¤H£½£«46.0 kJ”¤mol£1
H2(g) ¦¤H£½£«46.0 kJ”¤mol£1
ĻĀĮŠ¹ŲÓŚNH3(g)Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½µÄŹéŠ“ÕżČ·µÄŹĒ(””””)
A£®NH3(g)£«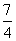 O2(g)===NO2(g)£«
O2(g)===NO2(g)£«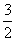 H2O(g) ¦¤H£½£282.8 kJ”¤mol£1
H2O(g) ¦¤H£½£282.8 kJ”¤mol£1
B£®NH3(g)£«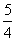 O2(g)===NO2(g)£«
O2(g)===NO2(g)£«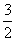 H2O(g) ¦¤H£½£161.9 kJ”¤mol£1
H2O(g) ¦¤H£½£161.9 kJ”¤mol£1
C£®NH3(g)£«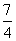 O2(g)===NO2(g)£«
O2(g)===NO2(g)£«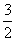 H2O(g) ¦¤H£½£161.9 kJ”¤mol£1
H2O(g) ¦¤H£½£161.9 kJ”¤mol£1
D£®NH3(g)£«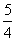 O2(g)===NO2(g)£«
O2(g)===NO2(g)£«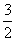 H2O(g) ¦¤H£½£282.8 kJ”¤mol£1
H2O(g) ¦¤H£½£282.8 kJ”¤mol£1
 ŗ®¼ŁŃ§ÓėĮ·ĻµĮŠ“š°ø
ŗ®¼ŁŃ§ÓėĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
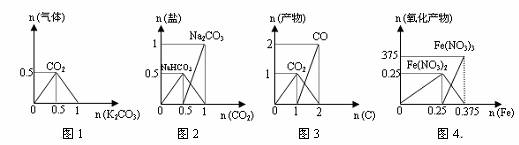
ĻĀĮŠĶ¼ĻóÄÜÕżČ·±ķŹ¾Ļą¹Ų·“Ó¦ÖŠ²śĪļĪļÖŹµÄĮæµÄ±ä»ÆµÄŹĒ£Øŗį”¢×Ż×ų±źµ„Ī»£ŗmol£©£Ø £©
A£®Ķ¼1£ŗn£ØHCl£©=1mol£¬K2CO3Öš²½¼ÓČėµ½HClČÜŅŗÖŠ£¬ŌŚ³ØæŚČŻĘ÷ÖŠÉś³ÉµÄĘųĢå
B£®Ķ¼2£ŗn£ØNaOH£©=1mol£¬CO2Öš²½ĶØČėµ½NaOHČÜŅŗÖŠ·“Ӧɜ³ÉµÄŃĪ
C£®Ķ¼3£ŗn£ØO2£©=1mol£¬øßĪĀĻĀCŗĶO2ŌŚĆܱÕČŻĘ÷ÖŠµÄ·“Ó¦²śĪļ
D£®Ķ¼4£ŗn£ØHNO3£©=1mol£¬FeŗĶĻ”HNO3·“Ӧɜ³ÉµÄŃõ»Æ²śĪļ£Ø»¹Ō²śĪļĪŖNO£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ4ÖÖĻ©Ģž·Ö±š¾“߻ƼÓĒāŗó²»ÄܵƵ½2¼×»łĪģĶéµÄŹĒ(””””)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖµØ·ÆČÜÓŚĖ®Ź±ČÜŅŗĪĀ¶Č½µµĶ”£µØ·Æ·Ö½āµÄČČ»Æѧ·½³ĢŹ½ĪŖCuSO4”¤5H2O(s)===CuSO4(s)£«5H2O(l)””¦¤H£½£«Q1 kJ”¤mol£1”£ŹŅĪĀĻĀ£¬Čō1 molĪŽĖ®ĮņĖįĶČܽāĪŖČÜŅŗ·ÅČČQ2 kJ£¬Ōņ(””””)””””””””””””””””””””””””””””””””””
A£®Q1>Q2 B£®Q1£½Q2 C£®Q1<Q2 D£®ĪŽ·Ø±Č½Ļ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖČČ»Æѧ·½³ĢŹ½£ŗ
(1)2H2O(l)===2H2(g)£«O2(g)
¦¤H£½£«571.6 kJ”¤mol£1
(2)2H2(g)£«O2(g)===2H2O(g)
¦¤H£½£483.6 kJ”¤mol£1
µ±1 gŅŗĢ¬Ė®±äĪŖĘųĢ¬Ė®Ź±£¬¶ŌĘäČČĮæ±ä»ÆÓŠĻĀĮŠĆčŹö£ŗ¢Ł·Å³ö£»¢ŚĪüŹÕ£»¢Ū2.44 kJ£»¢Ü4.88 kJ£»¢Ż88 kJ”£ĘäÖŠÕżČ·µÄŹĒ(””””)””””””””””””””””””””””””””””””””””””””
A£®¢ŚŗĶ¢Ż B£®¢ŁŗĶ¢Ū C£®¢ŚŗĶ¢Ü D£®¢ŚŗĶ¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŗģĮ×P(s)ŗĶCl2(g)·¢Éś·“Ӧɜ³ÉPCl3(g)ŗĶPCl5(g)”£·“Ó¦¹ż³ĢŗĶÄÜĮæ¹ŲĻµČēĻĀĶ¼ĖłŹ¾(Ķ¼ÖŠµÄ¦¤H±ķŹ¾Éś³É1 mol²śĪļµÄŹż¾Ż)”£
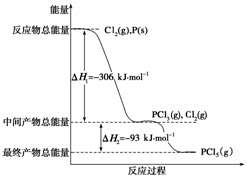
øł¾ŻÉĻĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ
(1) PŗĶCl2·“Ӧɜ³ÉPCl3µÄČČ»Æѧ·½³ĢŹ½ŹĒ
________________________________________________________________________
________________________________________________________________________ӣ
(2) PCl5·Ö½ā³ÉPCl3ŗĶCl2µÄČČ»Æѧ·½³ĢŹ½ŹĒ
________________________________________________________________________
________________________________________________________________________ӣ
ÉĻŹö·Ö½ā·“Ó¦ŹĒŅ»øöæÉÄę·“Ó¦”£ĪĀ¶ČT1Ź±£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČė0.80 mol PCl5£¬·“Ó¦“ļµ½Ę½ŗāŹ±PCl5»¹Ź£0.60 mol£¬Ęä¦Į1µČÓŚ________£»Čō·“Ó¦ĪĀ¶ČÓÉT1Éżøßµ½T2£¬Ę½ŗāŹ±PCl5µÄ·Ö½āĀŹĪŖ¦Į2£¬¦Į2________¦Į1(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)”£
(3) ¹¤ŅµÉĻÖʱøPCl5Ķس£·ÖĮ½²½½ųŠŠ£¬ĻČ½«PŗĶCl2·“Ӧɜ³ÉÖŠ¼ä²śĪļPCl3£¬Č»ŗó½µĪĀ£¬ŌŁŗĶCl2·“Ӧɜ³ÉPCl5”£ŌŅņŹĒ__________________________________________
______________________ӣ
(4)PŗĶCl2·ÖĮ½²½·“Ӧɜ³É1 mol PCl5µÄ¦¤H3£½______£¬PŗĶCl2Ņ»²½·“Ӧɜ³É1 mol PCl5µÄ¦¤H4________¦¤H3(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)”£
(5)PCl5Óė×ćĮæĖ®³ä·Ö·“Ó¦£¬×īÖÕÉś³ÉĮ½ÖÖĖį£¬Ęä»Æѧ·½³ĢŹ½ŹĒ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲŠŌÖŹµÄ±Č½Ļ£¬²»ÄÜÓĆŌŖĖŲÖÜĘŚĀɽāŹĶµÄŹĒ£Ø £©
A£®ĖįŠŌ£ŗH2SO4>H3PO4 B£®·Ē½šŹōŠŌ£ŗCl>Br
C£®¼īŠŌ£ŗNaOH>Mg(OH)2 D£®ČČĪČ¶ØŠŌ£ŗNa2CO3>NaHCO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀ±ķĪŖijӊ»śĪļÓėÖø¶ØŹŌ¼Į·“Ó¦µÄĻÖĻ󣬾Ż“ĖĶʶĻøĆÓŠ»śĪļŹĒ£Ø £©
| ŹŌ¼Į | ÄĘ | äåĖ® | ŠĀÖĘCu(OH)2 | ŅŅĖį |
| ĻÖĻó | ·Å³öĘųĢå | ĶŹÉ« | Öš½„Čܽā³ŹĄ¶É« | ²»·“Ó¦ |
A.CH2=CH£CHO B.CH2=CH£CH2OH
C. CH2=CH£COOH D.CH2=CH£CH2OCH3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ
A£®·Ö×ÓŹ½ĪŖC3H6ŗĶC6H12µÄĮ½ÖÖÓŠ»śĪļŅ»¶Ø»„ĪŖĶ¬ĻµĪļ
B£®Ļą¶Ō·Ö×ÓÖŹĮæĻąĶ¬µÄ²»Ķ¬ĪļÖŹŅ»¶ØŹĒĶ¬·ÖŅģ¹¹Ģå
C£®µķ·ŪŗĶĻĖĪ¬ĖŲµÄ·Ö×ÓŹ½ĻąĶ¬£¬ĖłŅŌĮ½Õß»„ĪŖĶ¬·ÖŅģ¹¹Ģå
D£®·Ö×ÓŹ½ĪŖC2H6ŗĶC5H12µÄĮ½ÖÖÓŠ»śĪļŅ»¶Ø»„ĪŖĶ¬ĻµĪļ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com