
| ||
| ||
| a |
| 17 |
| a |
| 17 |
| a |
| 17 |
| a |
| 17 |
| a |
| 34 |
| n |
| V |
| n(�����) | ||
|
| ||||
|
| 500ad |
| 40a+17b |
| 500ad |
| 40a+17b |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
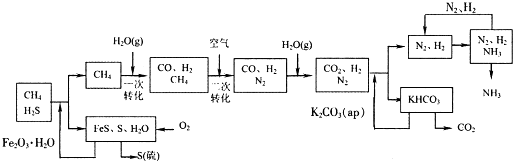
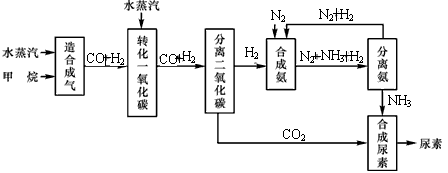
�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����
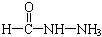
����д���пհף�
��1����֪0.5 mol������0.5 molˮ������t �桢p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
��2���ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ� ��
��3��������ϳɰ�����ת����Ϊ75%ʱ����5.60��107 L����Ϊԭ���ܹ��ϳɣ� L������������������ڱ�״���²ⶨ��
��4����֪���صĽṹ��ʽΪ![]() ��д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
��д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
�� ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��2 1.2�˹��̵�����-�ϳɰ���ϰ���������棩 ���ͣ������
�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����

����д���пհף�
��1����֪0.5 mol�����0.5 molˮ������t �棬p k Paʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� _____________
��2���ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʹ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪������������Ӧ�û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ� _____________________
��3��������ϳɰ�����ת����Ϊ75��ʱ����5.60��107 L����Ϊԭ���ܹ��ϳ� L ������������������ڱ�״���²ⶨ��
��4����֪���صĽṹ��ʽΪ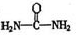 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����
����
��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����
����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ������
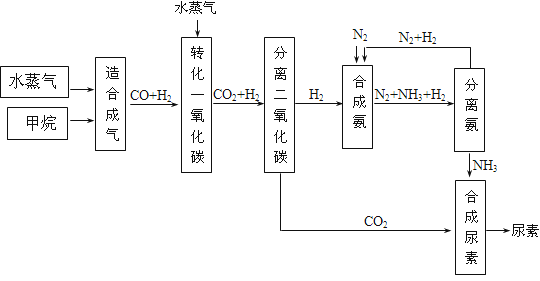
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
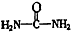 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����__________________ ����_______________________ ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����__________________ ����_______________________ �� �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com