
����Ŀ�����������ʾ��ij���е���Ҫ������Ⱦ��ΪSO2��NOx��CO�ȣ�����Ҫ��ԴΪȼú��������β�������������о���
��1��Ϊ����ȼú��SO2���ŷţ��ɽ�úת��Ϊ���ȼ��ˮú����CO��H2����
��֪��![]() ��H��241.8kJ��mol��1��
��H��241.8kJ��mol��1��
![]() ��H����110.5kJmol��1
��H����110.5kJmol��1
д����̿��1molˮ������Ӧ����ˮú�����Ȼ�ѧ����ʽ��________��
��2������β����NO���ڷ��������������ɵģ���ӦΪN2��g����O2��g��![]() 2NO��g�� ��H��0��
2NO��g�� ��H��0��
������0.8molN2��0.2molO2�����ƿ�����ɣ��Ļ���������ij�ܱ������У�����1300����Ӧ�ﵽƽ�⣬�������8��10��4molNO��������¶��´˷�Ӧ�Ļ�ѧƽ�ⳣ��K��________������Ƽ���������
�������������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ����________��
��3��������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�������������ų�����Һ����NO2��

���缫A�ĵ缫��ӦʽΪ________��
�缫B�ĵ缫��ӦʽΪ________��
�����������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����壬ͬʱ����SO32�����÷�Ӧ�����ӷ���ʽΪ________��
���𰸡�C��s����H2O��g����CO��g����H2��g�� ��H����131.3kJ��mol��14��10��6�¶����ߣ���Ӧ���ʼӿ���ƽ�������ƶ�SO2��2H2O��2e����SO42����4H��2HSO3����2H����2e����S2O42����2H2O4S2O42����2NO2��8OH����8SO32����N2��4H2O
��������(1)����֪����2H2(g)+O2(g)=2H2O(g)��H=-483.6KJ/mol����C(s)+![]() O2(g)�TCO(g)����H=-110.51kJmol-1�����ø�˹���ɣ�����-����
O2(g)�TCO(g)����H=-110.51kJmol-1�����ø�˹���ɣ�����-����![]() �ɵã�C(s)+H2O(g)=CO(g)+H2(g)����H=(-110.5kJ/mol)-(-241.8kJ/mol)=+13l.3 kJ/mol�����Խ�̿��ˮ������Ӧ���Ȼ�ѧ����ʽΪ��C(s)+H2O(g)�TCO(g)+H2(g)����H=+13l.30kJmol-1��
�ɵã�C(s)+H2O(g)=CO(g)+H2(g)����H=(-110.5kJ/mol)-(-241.8kJ/mol)=+13l.3 kJ/mol�����Խ�̿��ˮ������Ӧ���Ȼ�ѧ����ʽΪ��C(s)+H2O(g)�TCO(g)+H2(g)����H=+13l.30kJmol-1��
(2)���跴Ӧ�����������aL����������ã�
N2(g)+O2(g)![]() 2NO(g)
2NO(g)
��ʼŨ�� ![]()
![]() 0
0
ת��Ũ�� ![]()
![]()
![]()
ƽ��Ũ�� ![]() -
- ![]()
![]() -
- ![]()
![]()
���������ʵ�Ũ�ȴ������ʽ��K=![]() ��4��10-6��
��4��10-6��
�������з����Ļ�ѧ��Ӧ��N2(g)+O2(g)![]() 2NO(g)��H��0�����Ǹ����ȷ�Ӧ���¶����ߣ���Ӧ���ʼӿ죬ƽ�����������ƶ�����λʱ���ڲ�����NO�ࣻ
2NO(g)��H��0�����Ǹ����ȷ�Ӧ���¶����ߣ���Ӧ���ʼӿ죬ƽ�����������ƶ�����λʱ���ڲ�����NO�ࣻ
(3)������ͼʾ��֪��������������Ϊ����������Զ����������ڵ�A��Ϊ��������������������ӦSO2-2e-+2H2O�TSO42-+4H+��B��Ϊ��������HSO3������ԭΪS2O42���������ĵ缫��ӦΪ2HSO3����2H����2e����S2O42����2H2O��
�������ų�����ҺΪS2O42-�������������䷢����Ӧ��S2O



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ȷ£�CHCl3 �� �ǵ���ʣ��ڿ������ܷ����������������ɾ綾���ʹ�����COCl2 �� �ǵ���ʣ�����ѧ����ʽΪ2CHCl3+O2=2COCl2+2HCl�������ȷ��Ƿ�����Ӧѡ�õ��Լ��ǣ�������
A.ˮ
B.NaOH��Һ
C.��̪��Һ
D.�����ữ����������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ں���Cu��NO3��2��Mg��NO3��2��AgNO3����Һ�м�������п�ۣ������û������ǣ�������
A.Mg
B.Cu
C.Ag
D.H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʰ��յ��ʡ�������ᡢ��η���˳�����У�������ȷ���ǣ�������
A.ˮ�����ɱ������ᡢ�ռ����
B.��ơ��������ᡢ�����ơ�����
C.�����������������ᡢ�������ͭ
D.ͭ������ͭ�����ᡢ��ʯ�ҡ�ʯ��ʯ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L������Һ�����к��ȵIJⶨ��
������0.50 mol/L NaOH��Һ
��ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����_____g��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3 kJ/mol����_____________��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | |||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ______ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | ||
�ڽ�����Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c=4.18 J/(g�����������к��ȡ�H=______________ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�______��
A��ʵ��װ�ñ��¡�����Ч����
B����ȡNaOH��Һ�����ʱ���Ӷ���
C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������β���к���CO��NO2���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת���������塣
(1)����ȼ��(������ȼ�����������)��ͬʱ������β������Ҫ�ɷֲ�ͬ����ȼ�Ƚ�Сʱ���ж�������Ҫ��________(�ѧʽ)��
(2)ij����ɸ�����(H��������ʯ)��NH3�ѳ�NO��NO2�ķ�Ӧ������ͼ��ʾ��

�����ת����Ӧ�����ӷ���ʽ��________________������ͼ��Ӧ������NH3�ѳ�NO��NO2�ܷ�Ӧ�Ļ�ѧ����ʽ��_____________________________��
(3)NaClO2��Һ��NOת��ΪHNO3�ķ�Ӧ�������£�
NaClO2��HCl===HClO2��NaCl
8HClO2===6ClO2����Cl2����4H2O
2NaClO2��Cl2===2NaCl��2ClO2��
5NO��3ClO2��4H2O===5HNO3��3HCl
������������NaClO2��Һ��NOת��ΪHNO3���ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ӱ�����ǵ��������������е���������������������������������Ҫԭ��֮һ������������������������ж��ַ�����
(1)������Ԫ�طǽ����Խ�ǿ����________��
(2)NH3����ԭ��������(SCR)������ĿǰӦ����㷺���������������ѳ���������Ӧԭ����ͼ1��ʾ��

����ͼ1��֪SCR�����е�������Ϊ________��
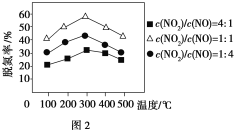
����Fe������ʱ���ڰ�������������£���ͬc(NO2)/c(NO)��Ӧ���ѵ�����ͼ2��ʾ���ѵ�Ч����ѵ�c(NO2)/c(NO)��________��
(3)��ˮ�������õ�����SO2�������������չ������£�
��SO2���ں�ˮ����H2SO3��H2SO3���ջ�����SO![]() ��SO
��SO![]() ���Ա���ˮ�е��ܽ�������ΪSO
���Ա���ˮ�е��ܽ�������ΪSO![]() ����ˮ��pH��________(����ߡ������䡱���͡�)��
����ˮ��pH��________(����ߡ������䡱���͡�)��
��SO2��O2��H2SO4��Һ�п��Թ���ԭ��أ��为����Ӧʽ��_____________________��
(4)��(Te)Ϊ�ڢ�A��Ԫ�أ��ǵ�����¼����²��ϵ���Ҫ�ɷ�֮һ����ҵ�Ͽɽ�SO2ͨ��TeCl4��������Һ�н��С���ԭ���õ��ڣ��÷�Ӧ�Ļ�ѧ����ʽ��___________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͬԪ�ص�ԭ���ڷ������������ӵ�������С����һ����ֵ x ����ʾ���� x Խ����ԭ��������������Խǿ�������γɵķ����г�Ϊ������ɵ�һ����
������ijЩ������Ԫ�ص� x ֵ��
Ԫ�ط��� | Li | Be | B | C | N | F |
x ֵ | 0.98 | 1.57 | 2.04 | 2.25 | 2.89 | 3.98 |
Ԫ�ط��� | Na | Mg | Si | P | S | Cl |
x ֵ | 0.93 | 1.35 | 1.90 | 2.19 | 2.58 | 3.16 |
��1��ͨ������ x ֵ�仯���ɣ�ȷ��Al��N�� x ֵ��Χ��________�� x (Al)��________
��2���Ʋ� x ֵ��ԭ�Ӱ뾶��ϵ��________�����ݶ�����Ԫ�ص� x ֵ�仯�ص㣬������Ԫ�����ʵ�________�仯���ɡ�
��3��ij�л�������ṹʽΪ�� ������SO��������Ϊ���õ��Ӷ�ƫ��˭��________��дԭ�����ƣ���
������SO��������Ϊ���õ��Ӷ�ƫ��˭��________��дԭ�����ƣ���
��4��������ɸ������ǣ����ɼ�����ԭ����ӦԪ�ص� x ��ֵ(�� x )�� x ��1.7ʱ��һ��Ϊ���Ӽ������� x ��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlF 3 �л�ѧ��������________��
��5��Ԥ��Ԫ�����ڱ��У� x ֵ��С��Ԫ�ص�λ�ã�_______��������Ԫ�س��⣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����д�ʩ��ʹˮ�ĵ���̶�������ǣ� ��
A. ����������Һ B. �Ӵ����� C. ���백ˮ D. ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com