


 CH3CH��CH3��CH2Br���ʴ�Ϊ��CH2=C��CH3��2+HBr
CH3CH��CH3��CH2Br���ʴ�Ϊ��CH2=C��CH3��2+HBr CH3CH��CH3��CH2Br��
CH3CH��CH3��CH2Br�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
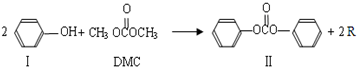
 ���ڴ���������Ҳ�����ɻ������ͬʱ�õ�һ�ָ���ƷG�������й�G��˵����ȷ����
���ڴ���������Ҳ�����ɻ������ͬʱ�õ�һ�ָ���ƷG�������й�G��˵����ȷ����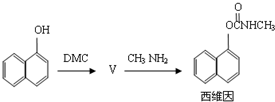
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����и߿�ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
̼���������DMC)��һ�����͵���ɫ�л��ϳ��м��壬���������о���ʹ�ð�ȫ�����㡢��Ⱦ�١�����������ص㡣��֪һ�������£�̼��������ܷ������·�Ӧ��
��Ӧ�٣�
��Ӧ�ڣ�
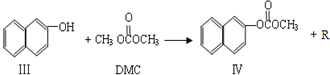
��1��������III�ķ���ʽΪ ����Ӧ�ٺͷ�Ӧ����R�Ľṹ��ʽΪ ��
��2��DMC������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
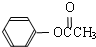
��3��̼������������ᱽ���� ���ڴ���������Ҳ�����ɻ������ͬʱ�õ�һ�ָ���ƷG�������й�G��˵����ȷ����_______��
���ڴ���������Ҳ�����ɻ������ͬʱ�õ�һ�ָ���ƷG�������й�G��˵����ȷ����_______��
A��G�������Ǽ�������
B�������G��Ϊͬ���칹��
C��һ�������£�G�ܷ���ˮ�ⷴӦ
D��G��������Cu(OH) 2��Ӧ���ɺ�ɫ����
��4����̼��������ϳ�ɱ�����ά���·�����£�

����������V�ķ�Ӧ�����ڷ�Ӧ�ڣ���V�Ľṹ��ʽΪ ��1mol��ά�������� mol H2�����ӳɷ�Ӧ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com