
��9�֣�������BN����һ����Ҫ�Ĺ����մɲ��ϡ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��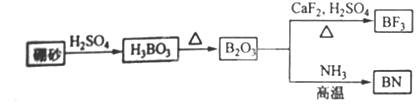
��ش��������⣺
��1����B2O3�Ʊ�BN��BF3�Ļ�ѧ����ʽ������______ ___��
___ _______��
��2��BF3����NaF���ÿ�����NaBF4��BF4��������ṹΪ____ ___��
��3������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ_____ _�����������Ϊ____ ____��
��4�������������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�Ӳ������ʯ�൱�������߳�Ϊ361.5pm��������������ܶ���____ ___g��cm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
| 25��4 |
| (361.5��10-101��)3NA |
| 25��4 |
| (361.5��10-101��)3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
 ����B3N3H6�Ķ��ȴ�����
����B3N3H6�Ķ��ȴ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| BF | - 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012��ɽ�����������ѧУ�߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
��9�֣�������BN����һ����Ҫ�Ĺ����մɲ��ϡ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��
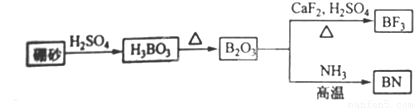
��ش��������⣺
��1����B2O3�Ʊ�BN��BF3�Ļ�ѧ����ʽ������______ ___��
___ _______��
��2��BF3����NaF���ÿ�����NaBF4��BF4��������ṹΪ____ ___��
��3������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ_ ___ _ _�����������Ϊ____ ____��
��4�������������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�Ӳ������ʯ�൱�������߳�Ϊ361.5pm��������������ܶ���____ ___g��cm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com