
H2ÓėCl2ŌŚµćČ¼»ņ¹āÕÕĢõ¼žĻĀ¶¼ÄÜ·¢Éś·“Ó¦”£
(1)H2ŌŚCl2ÖŠČ¼ÉÕŹ±µÄĻÖĻóĪŖ______________________________________£¬
øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________________________________________
________________________________________________________________________£¬
øĆ·“Ó¦ĪŖ________·“Ó¦(Ģī”°·ÅČČ”±»ņ”°ĪüČČ”±)”£
(2)ŅŃÖŖ¶ĻæŖ1 mol H2ÖŠµÄ»Æѧ¼üŠčĪüŹÕ436 kJµÄČČĮ棬¶ĻæŖ1 mol Cl2ÖŠµÄ»Æѧ¼üŠčĪüŹÕ243 kJµÄČČĮ棬¶ųŠĪ³É1 mol HCl·Ö×ÓÖŠµÄ»Æѧ¼üŹĶ·Å431 kJµÄČČĮ棬ŹŌĒó1 mol H2ŗĶ1 mol Cl2·“Ó¦µÄÄÜĮæ±ä»ÆĪŖ________kJ”£
½āĪö£ŗøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗH2£«Cl2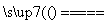 2HCl£¬¶ĻæŖ·“Ó¦ĪļÖŠµÄ»Æѧ¼üŠčĪüŹÕµÄ×ÜÄÜĮæĪŖ436 kJ£«243 kJ£½679 kJ£»ŠĪ³ÉÉś³ÉĪļÖŠµÄ»Æѧ¼üŹ±ŹĶ·ÅµÄ×ÜÄÜĮæĪŖ2”Į431 kJ£½862 kJ£¬¹ŹøĆ·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬ĒŅ·Å³ö862 kJ£679 kJ£½183 kJµÄČČĮ攣
2HCl£¬¶ĻæŖ·“Ó¦ĪļÖŠµÄ»Æѧ¼üŠčĪüŹÕµÄ×ÜÄÜĮæĪŖ436 kJ£«243 kJ£½679 kJ£»ŠĪ³ÉÉś³ÉĪļÖŠµÄ»Æѧ¼üŹ±ŹĶ·ÅµÄ×ÜÄÜĮæĪŖ2”Į431 kJ£½862 kJ£¬¹ŹøĆ·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬ĒŅ·Å³ö862 kJ£679 kJ£½183 kJµÄČČĮ攣
“š°ø£ŗ(1)H2ŌŚCl2ÖŠ°²¾²µŲČ¼ÉÕ£¬·¢³ö²Ō°×É«»šŃę””H2£«Cl2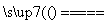 2HCl””·ÅČČ
2HCl””·ÅČČ
(2)183



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÖŠ²»ÕżČ·µÄŹĒ(””””)
A£®½šŹōŗĶ·Ē½šŹōŌ×ÓÖ®¼äŅ»¶ØŠĪ³ÉĄė×Ó¼ü
B£®Į½øö·Ē½šŹōŌ×ÓÖ®¼äŠĪ³ÉµÄ»Æѧ¼üŅ»¶ØŹĒ¹²¼Ū¼ü
C£®ŗ¬ÓŠĄė×Ó¼üµÄĪļÖŹ²»æÉÄÜŹĒµ„ÖŹ
D£®µ„ÖŹÖŠ²»Ņ»¶Øŗ¬ÓŠ¹²¼Ū¼ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠŅ»ÖÖæóŹÆ£¬²ā¶ØĘäŗ¬ÓŠµÄĆ¾”¢¹č”¢ŃõČżÖÖŌŖĖŲµÄÖŹĮæ±ČĪŖ12”Ć7”Ć16”£
(1)ÓĆŃĪµÄ×é³É±ķŹ¾Ęä»ÆѧŹ½£ŗ_____________________________________________£»
(2)ÓĆŃõ»ÆĪļµÄ×é³É±ķŹ¾Ęä»ÆѧŹ½£ŗ_________________________________________£»
(3)øĆæóŹÆµÄ³É·ÖŹōÓŚ________(ĢīĪļÖŹµÄ·ÖĄą)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
“Óŗ£Ė®ÖŠĢįČ”äåµÄ·½·ØÖ®Ņ»ŹĒ£ŗ
¢ŁĻņŗ£Ė®ÖŠĶØČėCl2£»
¢ŚĻņĖłµĆµÄČÜŅŗÖŠ¹ÄČėæÕĘų°Ńä哵³ö£»
¢ŪÓĆ“æ¼īČÜŅŗĪüŹÕäåÉś³ÉNaBr”¢NaBrO3£»
¢ÜŌŚÉĻŹöČÜŅŗÖŠ¼ÓČėĻ”ĮņĖįŹ¹äåĪö³ö£»
¢Ż¼ÓĘūÓĶ”¢Õńµ“”¢¾²ÖĆ”¢·ÖŅŗ”£
øł¾ŻŅŌÉĻŠšŹö£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³ö¢ŁÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ__________________________________________
____________________ӣ
(2)²½Öč¢ŚÖŠĄūÓĆĮĖäåµÄŠŌÖŹÓŠ____________________________________________
____________________ӣ
(3)¢ÜÖŠ·“Ó¦µÄŃõ»Æ¼ĮŹĒ______________£¬»¹Ō¼ĮŹĒ____________”£
(4)ÄÜ·ń½«¢ŻÖŠµÄĘūÓĶ»»³É¾Ę¾«£æ____________________________________
__________£¬ŌŅņŹĒ_____________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗ¢ŁÄÜĮæŌ½µĶµÄĪļÖŹ¾ĶŌ½ĪČ¶Ø£»¢Ś°×Į××Ŗ»Æ³ÉŗģĮ׏Ē·ÅČČ·“Ó¦”£¾Ż“Ė£¬ĻĀĮŠÅŠ¶Ļ»ņĖµ·ØÖŠÕżČ·µÄŹĒ(Ė«Ń”)(””””)
A£®ŌŚĻąĶ¬µÄĢõ¼žĻĀ£¬ŗģĮױȰ×Į×ĪȶØ
B£®ŌŚĻąĶ¬µÄĢõ¼žĻĀ£¬°×Į×±ČŗģĮ×ĪȶØ
C£®ŗģĮ×ŗĶ°×Į׵Ľį¹¹²»Ķ¬
D£®ŗģĮ×ČŻŅ×·¢Éś×ŌČ¼¶ų°×Į×Ōņ²»»į×ŌČ¼
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ
A£®³£ĪĀĻĀ£¬Čō“×ĖįÄĘÓė“×ĖįµÄ»ģŗĻČÜŅŗpH=7£¬ŌņC(Na+)=c(CH3COO-)
B£®Ä³ĪĀ¶ČĻĀ£¬pH=6µÄNaClČÜŅŗÖŠĄė×ÓÅØ¶ČµÄ“óŠ”¹ŲĻµ£ŗc(Na+)= c(Cl-)>c(H+)>c(OH-)
C£®Čō2a mol”¤L£1HCNÓėa mol”¤L£1 NaOHČÜŅŗµČĢå»ż»ģŗĻŗóĖłµĆČÜŅŗÖŠc(Na£«)>c(CN£)£¬Ōņ»ģŗĻČÜŅŗpH>7
D£®ŗ¬0.1 mol NaHCO3ŗĶ0.2molNa2CO3µÄ»ģŗĻŅŗÖŠ£ŗc(Na£«)£«c(H£«)£½c(OH£)£«c(HCO3”Ŗ)£«2c(CO32”Ŗ)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹµŃéÄÜ“ļµ½Ō¤ĘŚÄæµÄµÄŹĒ
| ±ąŗÅ | ŹµŃéÄŚČŻ | ŹµŃéÄæµÄ |
| A | ŹŅĪĀĻĀ£¬ÓĆpHŹŌÖ½·Ö±š²ā¶ØÅضČĪŖ0.1mol”¤L£1NaClOČÜŅŗŗĶ0.1mol”¤L£1CH3COONaČÜŅŗµÄpH | ±Č½ĻHClOŗĶCH3COOHµÄĖįŠŌĒæČõ |
| B | Ļņŗ¬ÓŠ·ÓĢŖµÄNa2CO3ČÜŅŗÖŠ¼ÓČėÉŁĮæBaC12¹ĢĢ壬ČÜŅŗŗģÉ«±äĒ³ | Ö¤Ć÷Na2CO3ČÜŅŗÖŠ“ęŌŚĖ®½āĘ½ŗā |
| C | Ļņ10mL 0.2 mol/L NaOHČÜŅŗÖŠµĪČė2µĪ0.1 mol/L MgCl2ČÜŅŗ£¬²śÉś°×É«³Įµķŗó£¬ŌŁµĪ¼Ó2µĪ0.1 mol/LFeCl3ČÜŅŗ£¬ÓÖÉś³ÉŗģŗÖÉ«³Įµķ | Ö¤Ć÷ŌŚĻąĶ¬ĪĀ¶ČĻĀµÄKsp£ŗ Mg(OH)2 £¾Fe(OH)3 |
| D | ·Ö±š²ā¶ØŹŅĪĀĻĀµČĪļÖŹµÄĮæÅØ¶ČµÄNa2SO3ÓėNa2CO3ČÜŅŗµÄpH£¬ŗóÕß½Ļ“ó | Ö¤Ć÷·Ē½šŹōŠŌS£¾C |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚĻĀĮŠø÷ČÜŅŗÖŠ£¬Ņ»¶ØÄÜ“óĮæ¹²“ęµÄŅ»×éĄė×ÓŹĒ
A£®ŗ¬ÓŠAlO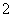 µÄČÜŅŗÖŠ£ŗNa£«”¢Al3£«”¢NO
µÄČÜŅŗÖŠ£ŗNa£«”¢Al3£«”¢NO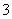 ”¢Cl£
”¢Cl£
B£®Ź¹µķ·Ūµā»Æ¼Ų±äĄ¶µÄČÜŅŗÖŠ£ŗK£«”¢HCO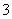 ”¢S2£”¢SO
”¢S2£”¢SO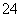
C£®ÄÜÓė½šŹōĀĮ·“Ó¦·Å³öĒāĘųµÄČÜŅŗÖŠ£ŗK£«”¢NO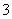 ”¢Cl£”¢Fe3£«
”¢Cl£”¢Fe3£«
D£®ĶøĆ÷ČÜŅŗÖŠ£ŗNa£«”¢MnO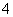 ”¢Cl£”¢SO
”¢Cl£”¢SO
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ×“Ģ¬µÄĪļÖŹ£¬¼ČŹōÓŚµē½āÖŹÓÖÄܵ¼µēµÄŹĒ(””””)
A£®¾Ę¾«”””””””””””””” B£®ĀČ»Æ¼Ų¾§Ģå
C£®ŅŗĀČ D£®ČŪČŚµÄĒāŃõ»ÆÄĘ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com