
��֪��A��B��F�Ǽ�ͥ�г������л��F������ʳƷ��װ��E��ʯ�ͻ�����չˮƽ�ı�־����������ת����ϵ�ش����⡣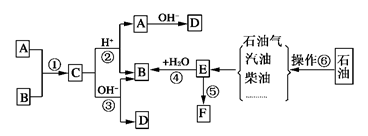
��1���ֱ�д��A��E�й����ŵ����ƣ�A�� ��E�� ��
(2) ����������Ϊ________________��
(3)ȡ����Ӧ�ķ���ܹ㣬�١���������ȡ����Ӧ����________(�����)��
(4)��д�����з�Ӧ�Ļ�ѧ����ʽ��
��.C��ϡ������Һ���ȷ�Ӧ______________________________________��
��.B�ڽ���ͭ�������ڿ����м��ȷ�Ӧ__________________________��
��.D��������ϡ���ᷴӦ____________________________________��
��.F�ڿ�������ȫȼ�� ��
(5)F��һ�ֳ����ĸ߷��Ӳ��ϣ��������Ǵ����˾�ķ��㡣Ȼ�������ֲ�����ɵĵ����ijһ����������________________________________________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
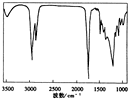 2008��9��13���������̨��������¹������ţ�������������谷ʹȫ������Ӥ����������ʯ�ġ���¹�̷��ش�ȫ�¹ʡ������� 22��Ӥ���̷�������ҵ��69���β�Ʒ����˺�����ͬ�������谷��Ϊ�˹���Ժ�������������ش�ʳƷ��ȫ�¹�I����Ӧ���ش������й������谷�����⣺
2008��9��13���������̨��������¹������ţ�������������谷ʹȫ������Ӥ����������ʯ�ġ���¹�̷��ش�ȫ�¹ʡ������� 22��Ӥ���̷�������ҵ��69���β�Ʒ����˺�����ͬ�������谷��Ϊ�˹���Ժ�������������ش�ʳƷ��ȫ�¹�I����Ӧ���ش������й������谷�����⣺
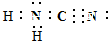
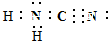
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
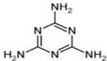
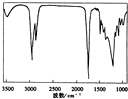
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ������ѧ�߶����£���ĩ��ѧģ���Ծ��������棩 ���ͣ������
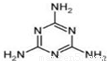
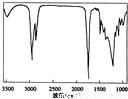
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com