
| �¶�/�� | 10 | 20 | 30 |
| ��NH4��2SO4 | 73.0 | 75.4 | 78.0 |
| FeSO4?7H2O | 20.0 | 26.5 | 32.9 |
| ��NH4��2SO4?FeSO4 | 17.2 | 21.6 | 28.1 |
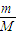 =
=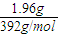 =0.005mol������ԭ���غ����������ӵ����ʵ���Ϊ0.005mol����Ӧ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O����5Fe2+��MnO4-�����Ը�����ص����ʵ���Ϊ0.001mol����c=
=0.005mol������ԭ���غ����������ӵ����ʵ���Ϊ0.005mol����Ӧ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O����5Fe2+��MnO4-�����Ը�����ص����ʵ���Ϊ0.001mol����c=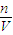 =
=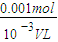 =
=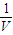 mol/l���ʴ�Ϊ��
mol/l���ʴ�Ϊ��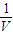 ��
��

 ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶�/�� | 10 | 20 | 30 | 40 | 50 | 70 |
| ��NH4��2SO4 | 73.0 | 75.4 | 78.0 | 81.0 | 84.5 | 91.9 |
| FeSO4?7H2O | 40.0 | 48.0 | 60.0 | 73.3 | - | - |
| ��NH4��2Fe��SO4��2?6H2O | 18.1 | 21.2 | 24.5 | 27.9 | 31.3 | 38.5 |
| 1 |
| V |
| 1 |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶�/�� | 10 | 20 | 30 |
| ��NH4��2SO4 | 73.0 | 75.4 | 78.0 |
| FeSO4?7H2O | 20.0 | 26.5 | 32.9 |
| ��NH4��2SO4?FeSO4 | 17.2 | 21.6 | 28.1 |

| 1 |
| V |
| 1 |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ʵ�黯ѧ��
��ʵ�黯ѧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶�/�� �ܽ��/g �� |
0 | 10 | 20 | 30 | 40 | 50 | 60 |
| ��NH4��2SO4 | 70.6 | 73.0 | 75.4 | 78.0 | 81.0 | - | 88.0 |
| FeSO4?7H2O | 15.7 | 20.5 | 26.5 | 32.9 | 40.2 | 48.6 | - |
| ��NH4��SO4FeSO4?6H2O | 12.5 | 17.2 | 21.6 | 28.1 | 33.0 | 40.0 | - |
| ��ʼ��������m1/g | ��Ӧ����������m2/g | Ī���ε����� | ���� | |
| ���۲���/g | ʵ�ʲ���m3/g | |||
| 5.0 | 2.2 | c | 14.7 | d |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��6 3.2 ���ʺ����IJⶨ��ϰ���������棩 ���ͣ������
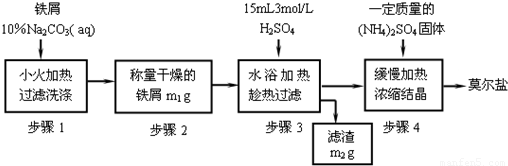
��������淋Ļ�ѧʽΪ(NH4)2SO4��FeSO4��6H2O������Ī���Σ��Ƿ�����ѧ�г����Ļ�ԭ����ij��ѧ�о�С���������ʵ�����Ʊ�Ī���β��ⶨ��������淋Ĵ��ȡ�
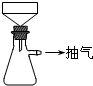
����һ����м�Ĵ������������ʢ��������м����ƿ�м���Na2CO3��Һ�����ȡ����ˡ�ϴ�ӡ����������������Ϊm1��
�������FeSO4���Ʊ�����������м���뵽һ������ϡ�����У���ַ�Ӧ����˲���������ˮϴ����ƿ����ֽ����Һ��ϴ��Һ��ȫת�����������С������������أ�������Ϊm2��
����������������淋��Ʊ���ȷ��ȡ����������(NH4)2SO4���롰��������е��������У���������һ��ʱ���ֹͣ����ȴ������������什ᾧ����ˡ���������ˮ�Ҵ�ϴ�Ӳ���Ȼ����������þ���������
�����ģ��ñ�ɫ���ⶨ��������淋Ĵ��ȡ�
�ش��������⣺
(1)�������г�ȡ��(NH4)2SO4����Ϊ________��
(2)����м��Na2CO3��Һ������Ŀ����_______________________________��
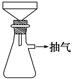
�Ʊ�FeSO4��Һʱ������ͼװ�ó��ȹ��ˣ�ԭ����
_______________________________________________________________��
�ڽ�(NH4)2SO4��FeSO4��Ϻ���ȡ�Ũ����ֹͣ���ȵ�ʱ����
________________________________________________________________________��
�۱�ɫ���ⶨ��������林��ȵ�ʵ�鲽��Ϊ��Fe3����ɫ�����ơ����������������Һ�����ơ���ɫ�ⶨ����ɫ�ʹ���Һ����ʱ�������������ϡ�����⣬��Ӧע���������________________________________________________________________________��
�ܸ�ʵ������ͨ��____________________ȷ����������鱗�Ʒ�ȼ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com