
æÕĘųÖŹĮæøßµĶÖ±½ÓÓ°Ļģ×ÅČĖĄąµÄÉś²śŗĶÉś»ī£¬ĖüŌ½Ą“Ō½ŹÜµ½ČĖĆĒµÄ¹Ų×¢”£±»ĪŪČ¾µÄæÕĘųÖŠŌÓÖŹµÄ³É·ÖÓŠ¶ąÖÖ£¬ĘäÖŠ¼ĘČė”¶æÕĘųÖŹĮæČձؔ·æÕĘųĪŪČ¾ÖøŹżµÄĻīÄæÓŠSO2”¢CO”¢NO2”¢O3ŗĶæÉĪüČėæÅĮ£ĪļµČ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©S”¢N”¢OµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņĪŖ ”£
£Ø2£©ŃŖŗģµ°°×ÖŠŗ¬ÓŠFe2£«£¬COŅ×ÓėŃŖŗģµ°°×½įŗĻ³ÉĪČ¶ØµÄÅäŗĻĪļ¶ųŹ¹ČĖÖŠ¶¾”£
¢ŁŠ“³öŃĒĢśĄė×ӵĻłĢ¬µē×ÓÅŲ¼Ź½ ”£
¢ŚCOæÉŅŌŗĶŗܶą¹ż¶É½šŹōŠĪ³ÉÅäŗĻĪļ”£CO·Ö×ÓÖŠCŌ×ÓÉĻÓŠŅ»¶Ō¹Ā¶Ōµē×Ó£¬C”¢OŌ×Ó¶¼·ūŗĻ8µē×ÓĪČ¶Ø½į¹¹£¬COÖŠÖŠŠÄŌ×ÓµÄŌÓ»ÆĄąŠĶĪŖ Ōӻƣ¬COÓŠ¶ąÖֵȵē×ÓĢ壬ĘäÖŠ³£¼ūµÄĮ½ÖÖĪŖ ”£
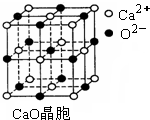
£Ø3£©SO2ŹĒŅ»ÖÖ“óĘųĪŪČ¾Īļ£¬ĪŖ¼õĒįSO2ĪŪČ¾£¬»šĮ¦·¢µē³§Éś²śÖŠ ³£ŌŚČ¼ĆŗÖŠ¼ÓČėCaOŅŌ”°¹ĢĮņ”±”£CaO¾§°ūČēĶ¼ĖłŹ¾£¬ĘäÖŠCa2+µÄÅäĪ»ŹżĪŖ £¬CaO¾§ĢåŗĶNaCl¾§ĢåÖŠĄė×ÓÅÅĮŠ·½Ź½ĻąĶ¬£¬Ę侧øńÄÜ·Ö±šĪŖ£ŗCaO”Ŗ3 401kJ/mol”¢NaCl”Ŗ786kJ/mol”£CaO¾§ĢåµÄČŪµć NaCl¾§ĢåµÄČŪµć£ØĢī”°øßÓŚ”±”¢”°µČÓŚ”±»ņ”°µĶÓŚ”±£©”£

£Ø4£©Ėę×ÅČĖĆĒÉś»īÖŹĮæµÄĢįøߣ¬²»½öŹŅĶāµÄ»·¾³°²Č«ĪŖČĖĆĒĖłÖŲŹÓ£¬ŹŅÄŚµÄ»·¾³°²Č«ŗĶŹ³Ę·°²Č«Ņ²Ō½Ą“Ō½ĪŖČĖĆĒĖł¹Ų×¢”£¼×Č©ŹĒŹŅÄŚÖ÷ŅŖæÕĘųĪŪČ¾ĪļÖ®Ņ»£ØĘä·ŠµćŹĒ£19.5”ę£©£¬¼×Č©ŹĒ”°¼Ł¾Ę”±ÖŠµÄÖ÷ŅŖÓŠŗ¦ĪļÖŹ£ØĘä·ŠµćŹĒ64.65”ę£©£¬1mol¼×Č©·Ö×ÓÖŠ![]() ¼üµÄŹżÄæĪŖ £¬¼×“¼µÄ·ŠµćĆ÷ĻŌøßÓŚ¼×Č©µÄÖ÷ŅŖŌŅņŹĒ ”£
¼üµÄŹżÄæĪŖ £¬¼×“¼µÄ·ŠµćĆ÷ĻŌøßÓŚ¼×Č©µÄÖ÷ŅŖŌŅņŹĒ ”£
 ĆūÅĘѧŠ£·Ö²ćÖÜÖܲāĻµĮŠ“š°ø
ĆūÅĘѧŠ£·Ö²ćÖÜÖܲāĻµĮŠ“š°ø »ĘøŌŗ£µķČ«³ĢÅąÓŲāŹŌ¾ķĻµĮŠ“š°ø
»ĘøŌŗ£µķČ«³ĢÅąÓŲāŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
æÕĘųÖŹĮæøßµĶÖ±½ÓÓ°Ļģ×ÅČĖĄąµÄÉś²śŗĶÉś»ī£¬ĖüŌ½Ą“Ō½ŹÜµ½ČĖĆĒµÄ¹Ų×¢”£±»ĪŪČ¾µÄæÕĘųÖŠŌÓÖŹµÄ³É·ÖÓŠ¶ąÖÖ£¬ĘäÖŠ¼ĘČė”¶æÕĘųÖŹĮæČձؔ·æÕĘųĪŪČ¾ÖøŹżµÄĻīÄæÓŠSO2”¢CO”¢NO2”¢O3ŗĶæÉĪüČėæÅĮ£ĪļµČ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©S”¢N”¢OµÄµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņĪŖ ”£
£Ø2£©SO2”¢CO”¢NO2”¢O3³£ĪĀĻĀ¾łĪŖĘųĢ壬¹ĢĢ¬Ź±¾łŹōÓŚ ¾§Ģ唣
£Ø3£©Ėę×ÅČĖĆĒÉś»īÖŹĮæµÄĢįøߣ¬ŹŅÄŚµÄ»·¾³°²Č«ŗĶŹ³Ę·°²Č«Ō½Ą“Ō½ĪŖČĖĆĒĖł¹Ų×¢”£¼×Č©£ØHCHO£©ŹĒŹŅÄŚÖ÷ŅŖæÕĘųĪŪČ¾ĪļÖ®Ņ»£ØĘä·ŠµćŹĒØC19.5 ”ę£©£¬¼×“¼£ØCH3OH£©ŹĒ”°¼Ł¾Ę”±ÖŠµÄÖ÷ŅŖÓŠŗ¦ĪļÖŹ£ØĘä·ŠµćŹĒ64.65 ”ę£©”£¼×Č©·Ö×ÓÖŠCŌ×Ó²ÉČ” ŌӻƹģµĄ·½Ź½”£¼×“¼µÄ·ŠµćĆ÷ĻŌøßÓŚ¼×Č©µÄÖ÷ŅŖŌŅņŹĒ£ŗ
__________ ӣ
£Ø4£©CuClµÄŃĪĖįČÜŅŗÄܹ»ÓėCO·¢Éś·“Ó¦£ŗCuCl+CO+H2O=Cu(CO)Cl”¤H2O£¬øĆ·“Ó¦æÉÓĆÓŚ²ā¶ØæÕĘųÖŠCOŗ¬Į攣
¢ŁŠ“³öĶŌ×ӵĻłĢ¬µē×ÓÅŲ¼Ź½ ”£
¢ŚCuClµÄ¾§Ģå½į¹¹ČēĻĀĶ¼¼×ĖłŹ¾£¬ÓėĶ¬Ņ»øöCl£¾ąĄė×ī½üµÄĻąĮŚCu£«ÓŠ øö”£
¢ŪCu(CO)Cl”¤H2OµÄ½į¹¹ČēÉĻĶ¼ŅŅĖłŹ¾£¬Ķ¼ÖŠ±źŹ¾³ö8øöŹĒ¹²¼Ū¼ü£¬ĘäÖŠ øöŹĒÅäĪ»¼ü£¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğÖŲĒģŹŠøßČż5ŌĀŌĀæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗŃ”ŌńĢā
æÕĘųÖŹĮæøßµĶÖ±½ÓÓ°Ļģ×ÅČĖĄąµÄÉś²śŗĶÉś»ī”£”¶æÕĘųÖŹĮæČձؔ·ÖŠæÕĘųĪŪČ¾ÖøŹżµÄĻīÄæÓŠSO2”¢ CO”¢NOx”¢O3ŗĶæÉĪüČėæÅĮ£ĪļµČ”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ £Ø £©
A£®“óĘųÖŠµÄ³ōŃõ²ć¶ŌµŲĒņÓŠŗÜŗƵı£»¤×÷ÓĆ£¬ĖłŅŌ“óĘųÖŠŗ¬ÓŠ“óĮæO3¶ŌČĖĢåÓŠŅę
B£®ŌŚ“߻ƼĮ×÷ÓĆĻĀ£¬COŗĶNOxæÉ×Ŗ»ÆĪŖĪŽ¶¾ĪļÖŹ
C£®ŃŖŗģµ°°×ÖŠŗ¬ÓŠFe2+£¬NO”¢COČŻŅ×ÓėŃŖŗģµ°°×½įŗĻ³ÉĪȶØĪļÖŹ¶ųŹ¹ČĖÖŠ¶¾
D£®SO2”¢NOxµÄ“óĮæÅÅ·Å»įµ¼ÖĀĖįÓźµÄ²śÉś
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğø£½ØŹ”ĘĪĢļŹŠøßČżŹŹÓ¦ŠŌĮ·Ļ°£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø13·Ö£©
æÕĘųÖŹĮæøßµĶÖ±½ÓÓ°Ļģ×ÅČĖĄąµÄÉś²śŗĶÉś»ī£¬ĖüŌ½Ą“Ō½ŹÜµ½ČĖĆĒµÄ¹Ų×¢”£±»ĪŪČ¾µÄæÕĘųÖŠŌÓÖŹµÄ³É·ÖÓŠ¶ąÖÖ£¬ĘäÖŠ¼ĘČė”¶æÕĘųÖŹĮæČձؔ·æÕĘųĪŪČ¾ÖøŹżµÄĻīÄæÓŠSO2”¢CO”¢NO2”¢O3ŗĶæÉĪüČėæÅĮ£ĪļµČ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©S”¢N”¢OµÄµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņĪŖ ”£
£Ø2£©SO2”¢CO”¢NO2”¢O3³£ĪĀĻĀ¾łĪŖĘųĢ壬¹ĢĢ¬Ź±¾łŹōÓŚ ¾§Ģ唣
£Ø3£©Ėę×ÅČĖĆĒÉś»īÖŹĮæµÄĢįøߣ¬ŹŅÄŚµÄ»·¾³°²Č«ŗĶŹ³Ę·°²Č«Ō½Ą“Ō½ĪŖČĖĆĒĖł¹Ų×¢”£¼×Č©£ØHCHO£©ŹĒŹŅÄŚÖ÷ŅŖæÕĘųĪŪČ¾ĪļÖ®Ņ»£ØĘä·ŠµćŹĒØC19.5 ”ę£©£¬¼×“¼£ØCH3OH£©ŹĒ”°¼Ł¾Ę”±ÖŠµÄÖ÷ŅŖÓŠŗ¦ĪļÖŹ£ØĘä·ŠµćŹĒ64.65 ”ę£©”£¼×Č©·Ö×ÓÖŠCŌ×Ó²ÉČ” ŌӻƹģµĄ·½Ź½”£¼×“¼µÄ·ŠµćĆ÷ĻŌøßÓŚ¼×Č©µÄÖ÷ŅŖŌŅņŹĒ£ŗ__________ ”£
£Ø4£©CuClµÄŃĪĖįČÜŅŗÄܹ»ÓėCO·¢Éś·“Ó¦£ŗCuCl+CO+H2O=Cu(CO)Cl”¤H2O£¬øĆ·“Ó¦æÉÓĆÓŚ²ā¶ØæÕĘųÖŠCOŗ¬Į攣
¢ŁŠ“³öĶŌ×ӵĻłĢ¬µē×ÓÅŲ¼Ź½ ”£
¢ŚCuClµÄ¾§Ģå½į¹¹ČēĻĀĶ¼¼×ĖłŹ¾£¬ÓėĶ¬Ņ»øöCl£¾ąĄė×ī½üµÄĻąĮŚCu£«ÓŠ øö”£

¢ŪCu(CO)Cl”¤H2OµÄ½į¹¹ČēÉĻĶ¼ŅŅĖłŹ¾£¬Ķ¼ÖŠ±źŹ¾³ö8øöŹĒ¹²¼Ū¼ü£¬ĘäÖŠ6øöŹĒÅäĪ»¼ü£¬ĒėŌŚĶ¼ÖŠÓĆ¼żĶ·±źŹ¾³ö”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com