

����д���пհף�
(1)Ӧѡ�õ�װ����(ֻҪ��д��ͼ��װ�õı��)____________��
(2)д��ʵ����Na2O2������Ӧ�Ļ�ѧ����ʽ��__________________________________��
(3)��ѡ��װ�õ�����˳��Ӧ��(����ӿڵ���ĸ�����ӽ���ʡ��)____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
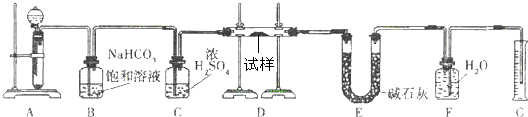
����һ��������Na2O���ʵ�Na2O2����������ͼ��ʵ��װ��ȷ��Na2O2�����Ĵ��ȡ�(�ɹ�ѡ�õķ�Ӧ��ֻ��CaCO3���塢6 mol/L���ᡢ6 mol/L���������ˮ)


�ش��������⣺
(1)ʵ��ǰ������װ�õ������ԡ��������Ӻú������������м�������ˮ���ر�A�з�Һ©�����ź����Թܣ���������������˵��װ�������Ժá�
(2)װ��A��Һ���Լ�ѡ��������������ᣬ����������������
(3)װ��B������������������
װ��C������������������
װ��E�м�ʯ�ҵ�����������������
(4)װ��D�з�����Ӧ�Ļ�ѧ����ʽ�ǣ� ��
(5)����ʼʱ�����Ʒ������Ϊ2.0 g����Ӧ���������������Ϊ224 mL(��״��)����Na2O2�����Ĵ���Ϊ������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com