
Ϊ���о�ͭ������ķ�Ӧ����ѧ��ȤС�����������ʵ��װ�ã�����a��b��c�ǻ�����
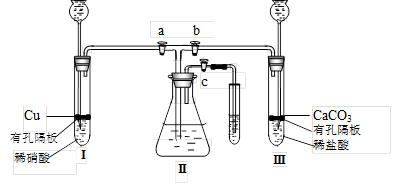 ��1��װҩƷǰ����ͼװ�����Ӻ���μ��������ԣ�
��1��װҩƷǰ����ͼװ�����Ӻ���μ��������ԣ�
��2����
ͼװҩƷ��������� a �� c ���ر�b ������е�������
����Ҫʹ���������ʼ�ձ�����ɫ��Ӧ������������
д�����з����ķ�Ӧ�����ӷ���ʽ��
��3�����������е�ϡ���ỻ��Ũ���ᣬ���� a �� c ���ر� b ����һ��۲죬���Կ�������û��ˮ�еĵ���������ð��������������ʺ���ɫ��Һ���Ϸ�����Ϊ��ɫ���������ּ�����ʽ������һ���� ��
��4����ʵ�飨2���У����װ�â���Һ���Ͽռ�������ΪVml���������������������Ϊ 21%��ʵ���ڱ�״���½��У�ȫ������ͨ����Ӧת��Ϊ���ʵ�鿪ʼʱ�����ͭΪmg��ʵ����Ϻ�ʣ��ͭng������������Һ�����ҲΪVml��������װ��������Ŀ��ܽ��룩�������������Һ�����ʵ���Ũ���� ��
��14�֣�
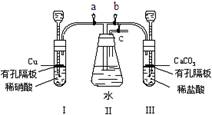
��1������һ����a��b��c�������ߵij���©���м�ˮ��û�˿ڣ�Ȼ���c��������ˮ���ڳ���©���ľ�������һ��ˮ�����۲�ˮ��Һ�棬���Һ�治�½����������Ժá�
����������a��c����b����I�м�ˮ��û����©���Ķ˿ڣ�Ȼ���c��������ˮ���ڳ���©���ľ�������һ��ˮ�����۲�ˮ��Һ�棬���Һ�治�½����������Ժã���b��c��a��ͬ���ϲ������װ��III�������ԡ�
��2�����ܿ�������ð����Һ��������ת��Ϊ����ɫ��
��a��b��c������һ����ʱ�䣬��II�еĿ���ȫ���ų���b��a��c��
3Cu+8H+ +2NO3-= 3Cu2+ + 2NO +4H2O
CaCO3+2H+=Ca2+ +CO2 +H2O
��3������ɫ��NO2��ˮ��Ӧ������ɫ��NO���塣3NO2+H2O��2HNO3+NO
��4��0.0125mol/L
����:


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
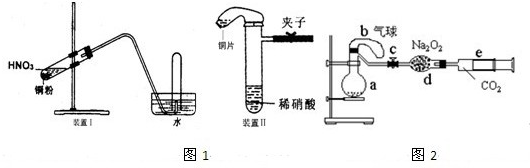
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ���о�ͭ������ķ�Ӧ����ѧ��ȤС�����������ʵ��װ�ã�����a��b��c�ǻ�����
��1��װҩƷǰ����ͼװ�����Ӻ���μ��������ԣ� ��2����ͼװҩƷ��������� a �� c ���ر�b ������е������� ����Ҫʹ���������ʼ�ձ�����ɫ��Ӧ������������ ��
��3�����������е�ϡ���ỻ��Ũ���ᣬ���� a �� c ���ر� b ����һ��۲죬���Կ�������û��ˮ�еĵ���������ð��������������ʺ���ɫ��Һ���Ϸ�����Ϊ��ɫ���������ּ�����ʽ������һ���� ��
��4����ʵ�飨2���У����װ�â���Һ���Ͽռ�������ΪVml���������������������Ϊ 21%��ʵ���ڱ�״���½��У�ȫ������ͨ����Ӧת��Ϊ���ʵ�鿪ʼʱ�����ͭΪmg��ʵ����Ϻ�ʣ��ͭng������������Һ�����ҲΪVml��������װ��������Ŀ��ܽ��룩�������������Һ�����ʵ���Ũ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2010�������Ӧ����2���ۻ�ѧ ���ͣ�ʵ����
Ϊ���о�ͭ������ķ�Ӧ����ѧ��ȤС�����������ʵ��װ�ã�����a��b��c�ǻ�����
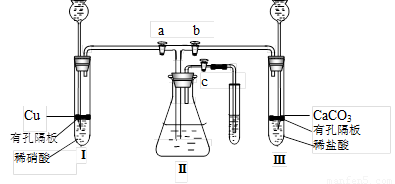 ��1��װҩƷǰ����ͼװ�����Ӻ���μ��������ԣ�
��1��װҩƷǰ����ͼװ�����Ӻ���μ��������ԣ�
��2����
ͼװҩƷ��������� a �� c ���ر�b ������е�������
����Ҫʹ���������ʼ�ձ�����ɫ��Ӧ������������
д�����з����ķ�Ӧ�����ӷ���ʽ��
��3�����������е�ϡ���ỻ��Ũ���ᣬ���� a �� c ���ر� b ����һ��۲죬���Կ�������û��ˮ�еĵ���������ð��������������ʺ���ɫ��Һ���Ϸ�����Ϊ��ɫ���������ּ�����ʽ������һ���� ��
��4����ʵ�飨2���У����װ�â���Һ���Ͽռ�������ΪVml���������������������Ϊ 21%��ʵ���ڱ�״���½��У�ȫ������ͨ����Ӧת��Ϊ���ʵ�鿪ʼʱ�����ͭΪmg��ʵ����Ϻ�ʣ��ͭng������������Һ�����ҲΪVml��������װ��������Ŀ��ܽ��룩�������������Һ�����ʵ���Ũ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ���о�ͭ������ķ�Ӧ����ѧ��ȤС�����������ʵ��װ�ã�����a��b��c�ǻ�����
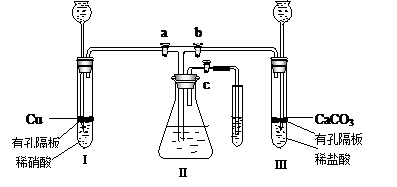 ��1��װҩƷǰ����ͼװ�����Ӻ���μ��������ԣ�
��1��װҩƷǰ����ͼװ�����Ӻ���μ��������ԣ�
��2����
ͼװҩƷ��������� a �� c ���ر�b ������е�������
����Ҫʹ���������ʼ�ձ�����ɫ��Ӧ������������
д�����з����ķ�Ӧ�����ӷ���ʽ��
��3�����������е�ϡ���ỻ��Ũ���ᣬ���� a �� c ���ر� b ����һ��۲죬���Կ�������û��ˮ�еĵ���������ð��������������ʺ���ɫ��Һ���Ϸ�����Ϊ��ɫ���������ּ�����ʽ������һ���� ��
��4����ʵ�飨2���У����װ�â���Һ���Ͽռ�������ΪVml���������������������Ϊ 21%��ʵ���ڱ�״���½��У�ȫ������ͨ����Ӧת��Ϊ���ʵ�鿪ʼʱ�����ͭΪmg��ʵ����Ϻ�ʣ��ͭng������������Һ�����ҲΪVml��������װ��������Ŀ��ܽ��룩�������������Һ�����ʵ���Ũ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com