
ijĪŽÉ«ČÜŅŗ£¬ĘäÖŠæÉÄÜ“ęŌŚNa£«”¢Ba2£«”¢AlO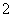 ”¢S2£”¢SO
”¢S2£”¢SO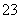 ”¢SO
”¢SO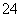 ”£Č”øĆČÜŅŗ½ųŠŠÓŠ¹ŲŹµŃ飬ŹµŃé½į¹ūČē×óĶ¼ĖłŹ¾”£
”£Č”øĆČÜŅŗ½ųŠŠÓŠ¹ŲŹµŃ飬ŹµŃé½į¹ūČē×óĶ¼ĖłŹ¾”£
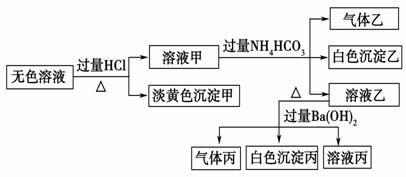
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)³Įµķ¼×µÄ»ÆѧŹ½ĪŖ________”£
(2)ÓÉČÜŅŗ¼×Éś³É³ĮµķŅŅµÄĄė×Ó·½³ĢŹ½ĪŖ______________”£
(3)³Įµķ±ūÖŠŅ»¶Øŗ¬ÓŠ___________(Ģī»ÆѧŹ½£¬ĻĀĶ¬)£¬æÉÄÜŗ¬ÓŠ__________”£
(4)×ŪŗĻÉĻŹöŠÅĻ¢£¬øĆČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓÓŠ_________”£
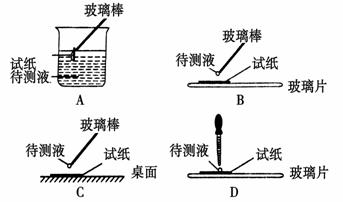 (5)øĆČÜŅŗĻŌŠŌ £ØĢīĖįŠŌ”¢¼īŠŌ»ņÖŠŠŌ£©£¬ČōŅŖ¼ģŃéĘäĖį¼īŠŌ£¬ĻĀĶ¼²Ł×÷ÕżČ·µÄŹĒ ”£
(5)øĆČÜŅŗĻŌŠŌ £ØĢīĖįŠŌ”¢¼īŠŌ»ņÖŠŠŌ£©£¬ČōŅŖ¼ģŃéĘäĖį¼īŠŌ£¬ĻĀĶ¼²Ł×÷ÕżČ·µÄŹĒ ”£
ĒėÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĻŌ¼īŠŌµÄŌŅņ£ŗ
 æģ½ŻÓ¢ÓļÖÜÖÜĮ·ĻµĮŠ“š°ø
æģ½ŻÓ¢ÓļÖÜÖÜĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ±ä»ÆŠčŅŖ¼ÓČėŹŹµ±µÄŃõ»Æ¼Į²ÅÄÜĶź³ÉµÄŹĒ( )”£
A£®CuO”śCu B£®Fe”śFeCl2 C£®H2SO4”śH2 D£®HNO3”śN2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦ŹōÓŚŃõ»Æ»¹Ō·“Ó¦µÄŹĒ
A£®CaCO3+2HCl=CaCl2+CO2”ü + H2O B£®2H2O2 2H2O+O2”ü
2H2O+O2ӟ
C£®CaO+H2O=Ca(OH)2 D£®CaCO3 CaO+CO2”ü
CaO+CO2ӟ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij¹¤³§µÄ¹¤Ņµ·ĻĖ®ÖŠŗ¬ÓŠ“óĮæµÄFeSO4”¢½Ļ¶ąµÄCu2+ŗĶÉŁĮæµÄNa+”£ĪŖĮĖ¼õÉŁĪŪČ¾²¢±ä·ĻĪŖ±¦£¬¹¤³§¼Ę»®“ÓøĆ·ĻĖ®ÖŠ»ŲŹÕĮņĖįŃĒĢśŗĶ½šŹōĶ”£Ēėøł¾ŻĮ÷³ĢĶ¼£¬ŌŚ·½æņŗĶĄØŗÅÄŚĢīŠ“ĪļÖŹĆū³Ę(»ņÖ÷ŅŖ³É·ÖµÄ»ÆѧŹ½)»ņ²Ł×÷·½·Ø£¬Ķź³É»ŲŹÕĮņĖįŃĒĢśŗĶĶµÄ¼ņµ„Źµ·½°ø
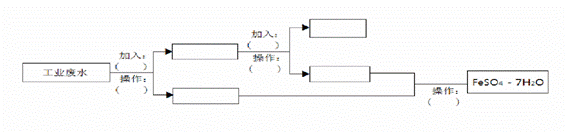
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖĻĀŹöČżøöŹµŃéÖŠµÄĪļÖŹ¾łÄÜ·¢Éś»Æѧ·“Ó¦”£
| ¢Ł | ¢Ś | ¢Ū |
| ½«Ģś¶¤·ÅČėĮņĖįĶČÜŅŗÖŠ | ĻņĮņĖįŃĒĢśČÜŅŗÖŠµĪČė¼øµĪÅØĻõĖį | ½«Ģś¶¤·ÅČėĀČ»ÆĢśČÜŅŗÖŠ |
ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ(””””)
A£®ŹµŃé¢ŁŗĶ¢ŪÖŠµÄĢś¶¤Ö»×÷»¹Ō¼Į
B£®ÉĻŹöŹµŃéÖ¤Ć÷Ńõ»ÆŠŌ£ŗFe3£«£¾Fe2£«£¾Cu2£«
C£®ŹµŃé¢ŚÖŠFe2£«¼ČĻŌŃõ»ÆŠŌÓÖĻŌ»¹ŌŠŌ
D£®ŹµŃé¢ŪÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖFe£«Fe3£«===2Fe2£«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĢõ¼žĻĀ£¬Ąė×ÓÄÜ“óĮæ¹²“ę»ņÕߥė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ
A£®pH=1µÄČÜŅŗÖŠ£ŗFe2+”¢ClO-”¢Na+”¢SO42-
B£®ŌŚŗ¬½Ļ¶ąAl3+µÄČÜŅŗÖŠ£ŗK+”¢Cl-”¢HCO3£
C£®NH4HSO4ČÜŅŗÓė¹żĮæNaOHĻ”ČÜŅŗ·“Ó¦£ŗNH4+ +OH££½NH3”¤H2O
D£®ĻņŠ”ĖÕ“ņČÜŅŗÖŠ¼ÓČėNaOH¹ĢĢå£ŗHCO3- + OH£=CO32- +H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĶ¬Ń§ÓūĢ½¾æŹ³Ę·Ģķ¼Ó¼Įļ§Ć÷·ÆNH4Al£ØSO4£©2”¤12H2OøßĪĀ·Ö½āµÄĒéæö”£
£Ø1£©Ō¤²ā²śĪļ£ŗĻĀĮŠ¹ŲÓŚĘųĢå²śĪļµÄŌ¤²ā²»ŗĻĄķµÄŹĒ ”£
A£®NH3”¢N2”¢SO2”¢H2O B£®NH3”¢SO3”¢H2O
C£®NH3”¢SO2”¢H2O D£®NH3”¢N2”¢SO3”¢SO2”¢H2O
£Ø2£©¶ØŠŌ¼ģŃé£ŗČ”Ņ»¶ØĮæļ§Ć÷·Æ£¬Éč¼ĘĻĀĮŠŹµŃéĢ½¾æ²śĪļ ”£
¢Ł°“Ķ¼Ź¾×é×°ŅĒĘ÷ŗó£¬Ź×ĻČ¼ģ²éÕūĢ××°ÖƵÄĘųĆÜŠŌ£¬²Ł×÷ŹĒ________”£
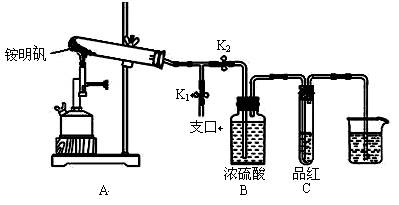
¢Ś¼Š×”Ö¹Ė®¼ŠK1£¬“ņæŖÖ¹Ė®¼ŠK2£¬ÓĆ¾Ę¾«ÅēµĘ³ä·Ö×ĘÉÕ”£ŹµŃé¹ż³ĢÖŠ£¬×°ÖĆAŗĶµ¼¹ÜÖŠĪ“¼ūŗģ×ŲÉ«ĘųĢ壻ŹŌ¹ÜCÖŠµÄĘ·ŗģČÜŅŗĶŹÉ«£»ŌŚÖ§æŚ“¦æɼģŃéµ½NH3£¬·½·ØŹĒ£»ŌŚ×°ÖĆAÓėBÖ®¼äµÄTŠĶµ¼¹ÜÖŠ³öĻÖ°×É«¹ĢĢ壬øĆ°×É«¹ĢĢåæÉÄÜŹĒ_________£ØČĪĢīŅ»ÖÖĪļÖŹµÄ»ÆѧŹ½£©”£
¢Ū·ÖĪöµĆ³ö×°ÖĆAŹŌ¹ÜÖŠ²ŠĮōµÄ°×É«¹ĢĢåŹĒĮ½ŠŌŃõ»ÆĪļ£¬Š“³öĖüČÜÓŚNaOHČÜŅŗµÄĄė×Ó·½³ĢŹ½”£
¢ÜĪŖĮĖ·ĄÖ¹µ¹Īü£¬ŹµŃé½įŹųŹ±±ŲŠėĻČ____________£ØĢī×ÖÄøŠņŗÅ£©£¬Č»ŗóĻØĆš¾Ę¾«ÅēµĘ”£
A£®Č”³öÉÕ±ÖŠµÄµ¼¹Ü B£®“ņæŖÖ¹Ė®¼ŠK1 C£®¹Ų±ÕÖ¹Ė®¼ŠK2
£Ø3£©·ÖĪöŗĶ½įĀŪ£ŗŹµŃéÖ¤Ć÷ĘųĢå²śĪļŹĒ£Ø1£©DÖŠµÄ5ÖÖĘųĢ唣ĻąĶ¬Ģõ¼žĻĀ²āµĆÉś³ÉN2ŗĶSO2µÄĢå»ż±ČŹĒ¶ØÖµ£¬V£ØN2£©£ŗV£ØSO2£©=____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚĖ®ČÜŅŗÖŠÄÜ“óĮæ¹²“ęµÄŅ»×éĄė×ÓŹĒ (””””)
A£®Ca2+”¢Al3+”¢Br-”¢CO3- B.Pb2+”¢Hg2+”¢S2-”¢SO3-
C. N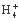 ӢH+ӢS2
ӢH+ӢS2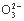 ӢP
ӢP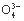 D.Na+ӢBa2+ӢCl-ӢNO3-
D.Na+ӢBa2+ӢCl-ӢNO3-
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
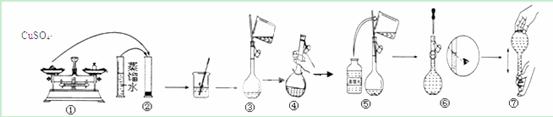
ŹµŃéŹŅĄļŠčŅŖÓĆ500mL 0.03 mol”¤L£1µÄĮņĖįĶČÜŅŗ”£
ŹŌ»Ų“šøĆČÜŅŗÅäÖĘ¹ż³ĢÖŠµÄĻĀĮŠĪŹĢā£ŗ
ŹµŃéŹŌ¼Į¼°ŅĒĘ÷£ŗĮņĖįĶ¾§Ģ唢ÕōĮóĖ®”¢ÉÕ±”¢ĢģĘ½”¢Ņ©³×”¢½ŗĶ·µĪ¹Ü”¢ĮæĶ²”£
£Ø1£©ŹµŃé»¹Č±ÉŁµÄŅĒĘ÷ÓŠ£ŗ ”£
£Ø2£©ĖłŠčµÄĮņĖįĶ¾§ĢåÖŹĮæĪŖ£ŗ g”£
£Ø3£©ĻĀĮŠ¶ŌČŻĮæĘæ¼°ĘäŹ¹ÓĆ·½·ØµÄĆčŹöÕżČ·µÄŹĒ£ŗ£Ø £©
a£®Ź¹ÓĆĒ°ŅŖ¼ģ²éČŻĮæĘæŹĒ·ńĀ©Ė®
b£®ČŻĮæĘæÉĻ±źĆ÷ĮĖ¹ęøń”¢ĪĀ¶ČŗĶÅضČ
c£®ČŻĮæĘæÓĆÕōĮóĖ®Ļ“¾»ŗó£¬ŌŁÓƱź×¼ĮņĖįĶČÜŅŗČóĻ“
d£®ÅäÖĘČÜŅŗŹ±£¬½«³ĘŗƵÄĮņĖįĶ¾§Ģ劔ŠÄµ¹ČėČŻĮæĘ棬¼ÓÕōĮóĖ®ÖĮ¾ąæĢ¶ČĻß1~2cm
”””” £Ø4£©ĻĀĶ¼ŹĒijĶ¬Ń§ÅäÖĘøĆ0.01 mol”¤L£1µÄĮņĖįĶČÜŅŗµÄ¹ż³ĢŹ¾ŅāĶ¼”£ĒėÄć¹Ū²ģĶ¼Ź¾ÅŠ¶ĻĘäÖŠ²»ÕżČ·µÄ²Ł×÷ÓŠ (ĢīŠņŗÅ)£»Čē¹ū°“ÕÕĶ¼Ź¾µÄ²Ł×÷ĖłÅäÖʵÄČÜŅŗ½ųŠŠŹµŃ飬ŌŚĘäĖū²Ł×÷¾łÕżČ·µÄĒéæöĻĀ£¬øĆĶ¬Ń§ĖłÅäÖʵÄĮņĖįĶČÜŅŗµÄÅØ¶Č½«
(Ģī”°Ę«“ó”±»ņ”°Ę«Š””±»ņ”°ĪŽ·ØČ·¶Ø”±)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com