
ЁОЬтФПЁПЛЏКЯЮяXЪЧвЛжжЯуСЯЃЌПЩВЩгУввЯЉгыМзБНЮЊжївЊдСЯЃЌАДЯТСаТЗЯпКЯГЩЃК
(1)аДГігЩввЯЉжЦШЁAЕФЛЏбЇЗНГЬЪНЃК______________________________________________________ЁЃ
(2)ввЯЉФмЪЙфхЫЎКЭЫсадKMnO4ШмвКЭЪЩЋЃЌЖўепЭЪЩЋдРэЯрЭЌТ№ЃП________ЁЃдвђЪЧ_____________________________________________________________________ЁЃ
(3)вдввЯЉЮЊдСЯЃЌФмЗёжЦЕУввШВЃП________ЁЃШєФмЃЌЧыаДГіЯрЙиЕФЛЏбЇЗНГЬЪНЃК_________________________________________________________________________________ЁЃ
(4)ЧыаДГіCЕФКЌгаБНЛЗЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃК___________________________________ЁЃ
(5)аДГіМзБНгыХЈЯѕЫсКЭХЈСђЫсЕФЛьКЯЫсЗДгІЕФЛЏбЇЗНГЬЪНЃК________________________ЁЃ
(6)вдМзБНЮЊР§ЫЕУїгаЛњЮяЛљЭХжЎМфЕФЯрЛЅгАЯьЃК_______________________ЁЃ
(7)аДГіCЁњDЕФЛЏбЇЗНГЬЪНЃК__________________________________________ЃЌЦфЗДгІРраЭЮЊ____________________________________ЁЃ
(8)CФмЗЂЩњЯћШЅЗДгІТ№ЃП________ЁЃдвђЪЧ__________________________________________ЁЃ
ЁОД№АИЁПCH2===CH2ЃЋH2O![]() CH3CH2OHВЛЭЌввЯЉЪЙфхЫЎЭЪЩЋЗЂЩњСЫМгГЩЗДгІЃЌЖјЪЙЫсадKMnO4ШмвКЭЪЩЋЗЂЩњбѕЛЏЗДгІФмCH2===CH2ЃЋBr2ЈDЁњ
CH3CH2OHВЛЭЌввЯЉЪЙфхЫЎЭЪЩЋЗЂЩњСЫМгГЩЗДгІЃЌЖјЪЙЫсадKMnO4ШмвКЭЪЩЋЗЂЩњбѕЛЏЗДгІФмCH2===CH2ЃЋBr2ЈDЁњ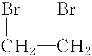 ЁЂ
ЁЂ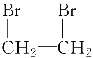 ЃЋ2NaOH
ЃЋ2NaOH![]() CHЁдCHЁќЃЋ2NaBrЃЋ2H2O
CHЁдCHЁќЃЋ2NaBrЃЋ2H2O
![]() ЃЋ3HNO3
ЃЋ3HNO3![]()
 ЃЋ3H2OМзБНЗжзгжаЁЊCH3КЭБНЛЗЯрЛЅгАЯьЃЌБНЛЗЖдМзЛљВњЩњгАЯьЃЌЪЙЕУЁЊCH3ПЩБЛЫсадKMnO4бѕЛЏЃЛЁЊCH3ЖдБНЛЗВњЩњгАЯьЃЌЪЙЁЊCH3СкЁЂЖдЮЛЧтдзгИќЛюЦУЁЂИќвзБЛШЁДњ
ЃЋ3H2OМзБНЗжзгжаЁЊCH3КЭБНЛЗЯрЛЅгАЯьЃЌБНЛЗЖдМзЛљВњЩњгАЯьЃЌЪЙЕУЁЊCH3ПЩБЛЫсадKMnO4бѕЛЏЃЛЁЊCH3ЖдБНЛЗВњЩњгАЯьЃЌЪЙЁЊCH3СкЁЂЖдЮЛЧтдзгИќЛюЦУЁЂИќвзБЛШЁДњ![]() ЃЋNaOH
ЃЋNaOH![]()
![]() ЃЋNaClЫЎНт(ШЁДњ)ЗДгІВЛФм
ЃЋNaClЫЎНт(ШЁДњ)ЗДгІВЛФм![]() жаТШдзгЯрСЌЬМдзгЕФСкЮЛЬМдзгЩЯЮоЧтдзг
жаТШдзгЯрСЌЬМдзгЕФСкЮЛЬМдзгЩЯЮоЧтдзг
ЁОНтЮіЁП
гЩЬтвтПЩжЊЃЌввЯЉгыЫЎдкДпЛЏМСЬѕМўЯТМгГЩЩњГЩввДМЃЌЙЪAЮЊввДМЃЌввДМНјвЛВНДпЛЏбѕЛЏЮЊBЃЌвђДЫBЮЊввЫсЃЌМзБНдкТШЦјЙтееЕФЬѕМўЯТЗЂЩњШЁДњЗДгІЩњГЩCЃЌвђДЫCЮЊвЛТШМзБН![]() ЃЌCдкЧтбѕЛЏФЦЫЎШмвКМгШШЬѕМўЯТЗЂЩњШЁДњЗДгІЃЌЙЪDЮЊ
ЃЌCдкЧтбѕЛЏФЦЫЎШмвКМгШШЬѕМўЯТЗЂЩњШЁДњЗДгІЃЌЙЪDЮЊ![]() ЃЌDКЭBЗЂЩњѕЅЛЏЗДгІЃЌЩњГЩFЃЌDДпЛЏбѕЛЏЮЊБНМзШЉЁЃ
ЃЌDКЭBЗЂЩњѕЅЛЏЗДгІЃЌЩњГЩFЃЌDДпЛЏбѕЛЏЮЊБНМзШЉЁЃ
ЃЈ1ЃЉввЯЉгыЫЎдкДпЛЏМСЕФЬѕМўЯТЛсЩњГЩввДМЃЌЛЏбЇЗНГЬЪНЮЊЃКCH2===CH2ЃЋH2O![]() CH3CH2OHЃЛ
CH3CH2OHЃЛ
ЃЈ2ЃЉввЯЉФмЪЙфхЫЎКЭЫсадKMnO4ШмвКЭЪЩЋЃЌЖўепЭЪЩЋдРэВЛЯрЭЌЃЌввЯЉФмЪЙфхЫЎЭЪЩЋЪЧЗЂЩњСЫМгГЩЗДгІЃЌввЯЉЪЙЫсадKMnO4ШмвКЭЪЩЋЃЌЪЧввЯЉБЛЫсадИпУЬЫсМиШмвКбѕЛЏЃЛ
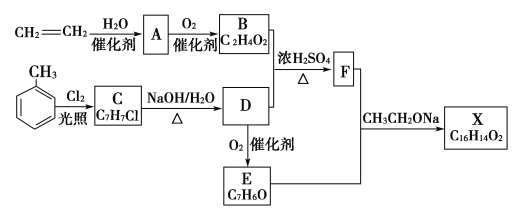
ЃЈ3ЃЉввЯЉгыфхЕЅжЪЗЂЩњМгГЩЗДгІЩњГЩ1,2-ЖўфхввЭщЁЂ1,2-ЖўфхввЭщдкЧтбѕЛЏФЦЕФДМШмвКвдМАМгШШЕФЬѕМўЯТЛсЗЂЩњЯћШЅЩњГЩввШВЃЌЛЏбЇЗНГЬЪНЮЊЃКCH2===CH2ЃЋBr2ЈDЁњ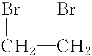 ЁЂ
ЁЂ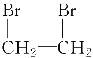 ЃЋ2NaOH
ЃЋ2NaOH![]() CHЁдCHЁќЃЋ2NaBrЃЋ2H2O ЃЛ
CHЁдCHЁќЃЋ2NaBrЃЋ2H2O ЃЛ
ЃЈ4ЃЉCЕФЛЏбЇЪНЮЊC7H7ClЃЌCЪЧгЩМзБНЗЂЩњШЁДњЗДгІЩњГЩЕФЃЌдђCЕФНсЙЙМђЪНЮЊ![]() ЃЌдђCДјБНЛЗЕФЭЌЗжвьЙЙЬхЮЊЃК
ЃЌдђCДјБНЛЗЕФЭЌЗжвьЙЙЬхЮЊЃК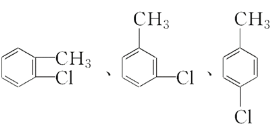 ЃЛ
ЃЛ
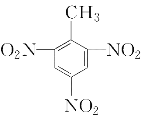
ЃЈ5ЃЉМзБНгыХЈЯѕЫсКЭХЈСђЫсЕФЛьКЯЫсЗЂЩњЗДгІЩњГЩШ§ЯѕЛљМзБНЃЌЛЏбЇЗНГЬЪНЮЊЃК![]() ЃЋ3HNO3
ЃЋ3HNO3![]()
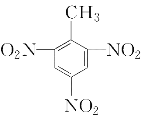 ЃЋ3H2O ЃЛ
ЃЋ3H2O ЃЛ
ЃЈ6ЃЉМзБНЗжзгжа-CH3КЭБНЛЗЯрЛЅгАЯьЃЌБНЛЗЖд-CH3ВњЩњгАЯьЃЌЪЙЕУ-CH3ПЩБЛЫсадKMnO4бѕЛЏЃЛ-CH3ЖдБНЛЗВњЩњгАЯьЃЌЪЙ-CH3СкЁЂЖдЮЛЧтдзгИќЛюЦУЁЂИќвзБЛШЁДњЃЛ
ЃЈ7ЃЉCЪЧгЩМзБНЗЂЩњШЁДњЗДгІЩњГЩЕФЃЌдђCЕФНсЙЙМђЪНЮЊ![]() ЃЌCдкЧтбѕЛЏФЦЕФЫЎШмвКжаЗЂЩњЫЎНтЗДгІЩњГЩБНМзДМЃЌЮЊЫЎНтЗДгІЃЌЫЎНтЗДгІвВЪєгкШЁДњЗДгІЃЌЛЏбЇЗНГЬЪНЮЊЃК
ЃЌCдкЧтбѕЛЏФЦЕФЫЎШмвКжаЗЂЩњЫЎНтЗДгІЩњГЩБНМзДМЃЌЮЊЫЎНтЗДгІЃЌЫЎНтЗДгІвВЪєгкШЁДњЗДгІЃЌЛЏбЇЗНГЬЪНЮЊЃК![]() ЃЋNaOH
ЃЋNaOH![]()
![]() ЃЋNaCl ЃЛ
ЃЋNaCl ЃЛ
ЃЈ8ЃЉCЪЧгЩМзБНЗЂЩњШЁДњЗДгІЩњГЩЕФЃЌдђCЕФНсЙЙМђЪНЮЊ![]() ЃЌЪєгкТБДњЬўЃЌЕЋЪЧгЩгк
ЃЌЪєгкТБДњЬўЃЌЕЋЪЧгЩгк![]() жаТШдзгЯрСЌЬМдзгЕФСкЮЛЬМдзгЩЯЮоЧтдзгЃЌВЛФмЗЂЩњЯћШЅЗДгІЁЃ
жаТШдзгЯрСЌЬМдзгЕФСкЮЛЬМдзгЩЯЮоЧтдзгЃЌВЛФмЗЂЩњЯћШЅЗДгІЁЃ


 ГЄНзївЕБОЭЌВНСЗЯАВсЯЕСаД№АИ
ГЄНзївЕБОЭЌВНСЗЯАВсЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЬМКЭЕЊЪЧЖЏжВЮяЬхжаЕФживЊзщГЩдЊЫиЃЌЯђДѓЦјжаЙ§ЖШХХЗХЖўбѕЛЏЬМЛсдьГЩЮТЪваЇгІЃЌЕЊбѕЛЏЮяЛсВњЩњЙтЛЏбЇбЬЮэЃЌФПЧАЃЌетаЉгаЖОгаКІЦјЬхЕФДІРэГЩЮЊПЦбЇбаОПЕФживЊФкШнЁЃ
ЃЈ1ЃЉвбжЊШШЛЏбЇЗНГЬЪНЃКЂй2C2H2(g)ЃЋ5O2(g)===4CO2(g)ЃЋ2H2O(l)ЁЁІЄH1
ЂкC(s)ЃЋO2(g)===CO2(g)ЁЁІЄH2
ЂлH2(g)ЃЋ1/2O2(g)===H2O(l)ЁЁІЄH3
дђЗДгІЂм2C(s)ЃЋH2(g)===C2H2(g)ЕФІЄH=_________ЁЃ(гУКЌІЄH1ЁЂІЄH2ЁЂІЄH3ЕФЙиЯЕЪНБэЪО)
ЃЈ2ЃЉРћгУЩЯЪіЗДгІЂйЩшМЦШМСЯЕчГиЃЈЕчНтжЪШмвКЮЊЧтбѕЛЏМиШмвКЃЉЃЌаДГіЕчГиЕФИКМЋЗДгІЪНЃК__________________________________________ ЁЃ
ЃЈ3ЃЉгУЛюадЬПЛЙдЗЈПЩвдДІРэЕЊбѕЛЏЮяЁЃФГбаОПаЁзщЯђФГУмБеШнЦїжаМгШывЛЖЈСПЕФЛюадЬПКЭNOЃЌЗЂЩњЗДгІC(s)ЃЋ2NO(g)![]() N2(g)ЃЋCO2(g)ЁЁІЄH<0ЁЃдкT1ЁцЪБЃЌЗДгІНјааЕНВЛЭЌЪБМфВтЕУИїЮяжЪЕФХЈЖШШчЯТЃК
N2(g)ЃЋCO2(g)ЁЁІЄH<0ЁЃдкT1ЁцЪБЃЌЗДгІНјааЕНВЛЭЌЪБМфВтЕУИїЮяжЪЕФХЈЖШШчЯТЃК
ЪБМф/min ХЈЖШ/(molЁЄLЃ1) ЮяжЪ | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.00 | 0.68 | 0.50 | 0.50 | 0.60 | 0.60 |
N2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
CO2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
Ђй10ЁЋ20 minФкЃЌNOЕФЦНОљЗДгІЫйТЪv(NO)ЃН______ЃЌT1ЁцЪБЃЌИУЗДгІЕФЦНКтГЃЪ§KЃН________ЁЃ
Ђк30 minКѓЃЌжЛИФБфФГвЛЬѕМўЃЌЗДгІжиаТДяЕНЦНКтЃЌИљОнЩЯБэжаЕФЪ§ОнХаЖЯИФБфЕФЬѕМўПЩФмЪЧ________(ЬюзжФИБрКХ)ЁЃ
aЃЎЭЈШывЛЖЈСПЕФNO bЃЎМгШывЛЖЈСПЕФC cЃЎЪЪЕБЩ§ИпЗДгІЬхЯЕЕФЮТЖШ
dЃЎМгШыКЯЪЪЕФДпЛЏМС eЃЎЪЪЕБЫѕаЁШнЦїЕФЬхЛ§
ЂлШєБЃГжгыЩЯЪіЗДгІЧА30 minЕФЗДгІЬѕМўЯрЭЌЃЌЦ№ЪМЪБNOЕФХЈЖШЮЊ2.50 molЁЄLЃ1ЃЌдђЗДгІДяЦНКтЪБc(NO)ЃН________ЃЌNOЕФзЊЛЏТЪЃН________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЪєгкЧПЕчНтжЪЕФЪЧЃЈ ЃЉ
A.ЬМЫсИЦB.ЪГбЮЫЎC.АБЦјD.ДзЫс
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУДПОЛЕФCaCO3гы100mLЯЁбЮЫсЗДгІжЦШЁCO2ЃЌЪЕбщЙ§ГЬМЧТМШчЭМЫљЪО(ЦјЬхЬхЛ§вбелЫуЮЊБъзМзДПіЯТЕФЬхЛ§)ЁЃЯТСаЗжЮіе§ШЗЕФЪЧЃЈ ЃЉ
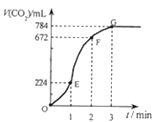
A. OEЖЮБэЪОЕФЦНОљЫйТЪзюПь
B. EFЖЮЃЌгУбЮЫсБэЪОИУЗДгІЕФЦНОљЗДгІЫйТЪЮЊ 0.4mol/(LЁЄmin)
C. OEЁЂEFЁЂFGШ§ЖЮжаЃЌгУЖўбѕЛЏЬМБэЪОИУЗДгІЕФЦНОљЗДгІЫйТЪжЎБШЮЊ2:6:7
D. GЕуCO2ВЛдйдіМгЕФдвђПЩФмЪЧбЮЫсвбгУЭъ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУЯТСазАжУНјааЪЕбщЃЌФмДяЕНЯргІЪЕбщФПЕФЕФЪЧ

A. ЭММзПЩгУгкеєСѓЪЏгЭВЂЪеМЏ60ЁЋ150ЁцСѓЗж
B. ЭМввПЩгУгкГ§ШЅввЯЉЦјЬхжаЛьгаЕФЩйСПSO2
C. ЭМБћзАжУПЩгУгкБШНЯSЁЂCЁЂSiШ§жждЊЫиЕФЗЧН№Ъєад
D. ЭМЖЁзАжУПЩгУгкВтЖЈCO2ЕФЩњГЩЫйТЪ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдквЛУмБеШнЦїжаЗЂЩњЗДгІЃК2A(g)ЃЋ2B(g)![]() C(s)ЃЋ3D(g)ЁЁІЄHЃМ0ЃЌДяЕНЦНКтЪБВЩШЁЯТСаДыЪЉЃЌПЩвдЪЙе§ЗДгІЫйТЪvе§діДѓЁЂDЕФЮяжЪЕФСПХЈЖШc(D)діДѓЕФЪЧ(ЁЁЁЁ)
C(s)ЃЋ3D(g)ЁЁІЄHЃМ0ЃЌДяЕНЦНКтЪБВЩШЁЯТСаДыЪЉЃЌПЩвдЪЙе§ЗДгІЫйТЪvе§діДѓЁЂDЕФЮяжЪЕФСПХЈЖШc(D)діДѓЕФЪЧ(ЁЁЁЁ)
A. вЦзпЩйСПC B. РЉДѓШнЛ§ЃЌМѕаЁбЙЧП
C. ЫѕаЁШнЛ§ЃЌдіДѓбЙЧП D. ШнЛ§ВЛБфЃЌГфШыЁАЖшЁБЦј
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЪТЪЕгыбЮРрЫЎНтЮоЙиЕФЪЧЃЈ ЃЉ
A.УїЗЏПЩвдОЛЫЎ
B.ЪЙгУШШЕФДПМюШмвКШЅГ§гЭЮлаЇЙћКУ
C.ЪЕбщЪвХфжЦ FeCl2 ШмвКЪБМгШыЩйСПЯЁбЮЫс
D.Яђ FeCl3 ШмвКжаМгШы KOH ШмвКВњЩњКьКжЩЋГСЕэ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЮяжЪМгШыЫЎжаЪєгкЗХШШЗДгІЕФЪЧ
A.ЩњЪЏЛвB.ЙЬЬхNaOHC.ХЈСђЫсD.ЙЬЬхNH4NO3
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЭЈЙ§вдЯТЗДгІПЩЛёЕУаТаЭФмдДЖўМзУбЃЈCH3OCH3)ЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
ЂйC(s) + H2O(g)==CO(g)+H2(g) ІЄH1=a kJmol-1
ЂкCO(g) + H2O(g)==CO2(g)+H2(g) ІЄH2=b kJmol-1
ЂлCO2(g)+3H2(g)==CH3OH(g)+H2O(g) ІЄH3 =c kJmol-1
Ђм2CH3OH(g) ==CH3OCH3(g)+H2O(g) ІЄH4=d kJmol-1
A. ЗДгІЂйЂкЮЊЗДгІЂлЬсЙЉдСЯЦј
B. ЗДгІЂлвВЪЧCO2зЪдДЛЏРћгУЕФЗНЗЈжЎвЛ
C. ЗДгІCH3OH(g)== ![]() CH3OCH3(g)+
CH3OCH3(g)+ ![]() H2O(1)ЕФІЄH =
H2O(1)ЕФІЄH =![]() kJmol-1
kJmol-1
D. ЗДгІ 2CO(g)+4H2(g) ==CH3OCH3(g)+H2O(g)ЕФІЄH= (2b+2c+d) kJmol-1
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com