
ij��ѧ��ȤС����ֻ������������ͭ�Ĺ�ҵ������ȡ�������Ȼ�����Һ���̷�����(FeSO4��7H2O)�͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�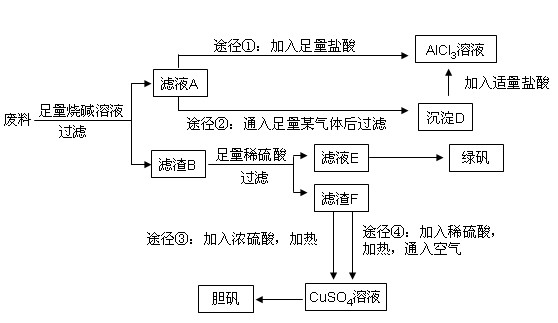
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ�� ��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�;������ͨ���ij���壨��̬ʱ�������˹����꣩��д��������ĵ���ʽ ������Ϊ�Ϻ�����;���� ����ٻ�ڣ��������ǣ� ��
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ���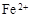 ��
��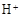 �⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
�⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
��4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��ǣ� �� ��
��5��;���ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��6��ʵ���Ҵ�CuSO4��Һ��ȡ��������������������Ũ������ȴ�ᾧ�� ����Ȼ���
��1��2Al +2OH�D + 2H2O = 2AlO2�D +3H2�� (2��)
��2�� (1��) �� (1��) ;������ȡ��AlCl3��Һ�л���NaCl����(1��)
(1��) �� (1��) ;������ȡ��AlCl3��Һ�л���NaCl����(1��)
��3��Fe3�� (1��) ȡ�����μ�KSCN������Ѫ��ɫ������Fe3�� ��(1��)
��4���ɱ��� (1�֣��������֣���ͬ) ���������ж����� (1��)
��5��2Cu +2H2SO4 +O2�� 2CuSO4 +2H2O (2��)
��6������ϴ�� (1��)
�������������
��1����������ֻͭ�������ռ���Һ��Ӧ��
��2����NaAlO2��Ӧ����Al(OH)3����̬ʱ�������˹����꣬������CO2 ��NaAlO2 +4HCl=AlCl3+NaCl+2H2O ����AlCl3��Һ�л���NaCl���ʡ�
��3��Fe2��������������Fe3����Fe3����KSCN��Ӧ����Ѫ��ɫ��Fe2�����ܡ�
��4��;����Cu+2H2SO4= CuSO4+ SO2��+2H2O
;���� 2Cu +2H2SO4 +O2 = 2CuSO4 +2H2O;������ϡH2SO4�ɱ��ͣ��������ж�����
���㣺�����Ի�ѧʵ��Ϊ����������Ԫ�ؼ��仯�����ʵ���֪ʶ��


 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ȼ�ͭ��һ�ֹ㷺�����������ϡ���ľ�ķ������Ļ�����Ʒ��ij�о���ѧϰС���ô�ͭ��������Fe�������������Ʊ��Ȼ�ͭ���塣
��1������A��ϡ�����ܽ������ˮ�ܽ��ԭ����________��
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ�X��ѡ�������Լ��е�____������ţ���
| A��NaOH | B��NH3.H2O | C��CuO | D��Cu(OH)2E. CuSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���÷ϵ�����˿�����������⣩������ͭ��Һ(����������)�ͱ��л�����Ⱦ�ķ�ͭ���Ʊ�����ͭ���塣������������ͼ��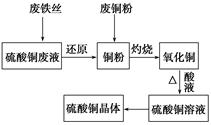
�Իش��������⣺
(1)��˿��Ͷ������ͭ��Һǰ����ϡH2SO4���д�������Ŀ����__________________�����ܷ�����Ӧ�����ӷ���ʽ��Fe��2H��=Fe2����H2����_____________________��__________________��
(2)��ͭ���뻹ԭ����ͭ�ۻ�����գ����鷢�����պ�õ�����CuO������Cu�Ļ���ԭ���ǣ�
�����ղ����Cuδ����ȫ������
��CuOδ����ԭ����ԭ����_______________��
(3)Ϊ��ʹ���պ�Ļ���������ܣ��ڼ���ϡH2SO4��ͬʱ��Ҳ����������H2O2��Һ����������50��60�棬������Ӧ1h����ش��������⣺
�ٷ�Ӧʱ�¶ȱ��������50��60�棬�¶Ȳ��˹��ߣ�����������Ŀ��_____��
��д����Ӧ�Ļ�ѧ����ʽ��_____________��________________________________��
(4)������ͭ��Һ�õ�����ͭ�����ʵ�����Ϊ________________________��
(5)ֱ�������պ�Ļ�����м���Ũ���Ტ���Ƚ������ܣ�Ҳ�ɴﵽ������ܵ�Ŀ�ģ���ʵ�ʲ����н���ʹ�ã�ԭ�������_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ZnO���п����ԣ�Ҳ����Ҫ����̥���Ӽ�����ҵ���ɴ�ZnO(��FeO��CuO)�Ʊ�����ZnO�������£� 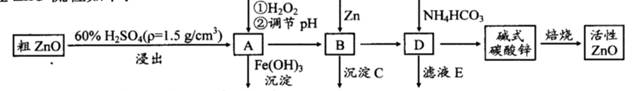
��֪����Һ��Fe2+��Fe3+��Cu2+��Zn2+�γ����������pH���±���
| ���� | ��ʼ������pH | ��ȫ������pH |
| Fe2+ | 6.4 | 8.4 |
| Fe3+ | 2.4 | 3.1 |
| Cu2+ | 5.2 | 6.7 |
| Zn2+ | 6.8 | 9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ��(CuFeS2)����ȡͭ���仯�������Ҫԭ��֮һ������¯������Ҫ�ɷ� ��FeO��Fe2O3��SiO2��Al2O3��������������ת����ϵ,��ش�
��1��д����֤��SO2�������������������ԵĻ�ѧ����ʽ________________��
��2����NaOH��Һ����SO2����NaHSO3��ҺpH��7�������Һ�д������ӵ����ʵ���Ũ���ɴ�С��˳����________��
��3��д��Cu2S������ȡ��ͭ�Ļ�ѧ����ʽ________________________
��4���ϵ��Һ�г�����Pb2+��Zn2+����ϵ��Һ�м���Na2S��Һ������PbS��ZnS����ʱ��C(Zn2+): C(Pb2+)��________��[��֪��Ksp(PbS)��3.4��10��28mol2��L��2��Ksp(ZnS)��1.6��10��24mol2��L��2��]
��5��д��֤����ҺI�к���Fe2+��ʵ�����________________��
��6��Na2FeO4��ɱ����ˮ��ԭ����________________��
��7��Na2FeO4��Zn������ɼ��Ե�أ��䷴ӦʽΪ:3Zn+2FeO42-+8H2O��3Zn(OH)2+2Fe(OH)+4OH-����д���ŵ�ʱ�����缫��Ӧʽ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ��һ���������ϵ�dz����е���ɫ�����������£�Cu2+����Һ���ȶ���Cu+�����������������绯��Ӧ��2Cu+=Cu2++Cu�������+1��ͭ�Ļ�������������磺Cu20��Cul��CuCl��CuH �ȡ�
��1����CuCl2��Һ����μ������KI��Һ�����ܷ����ķ�Ӧ�У�
2Cu2++4I-=2CuI������ɫ��+I2; 2Cu2++4I-+2Cl-=CuCl������ɫ��+I2
��֪��������Ksp(CuCl)=1.20��10-6(mol/L)2; Ksp(CuI)=5.06��10-12(mol/L)2���ɴ��ƶ�������Ӧ������Ҫ������Ļ�ѧʽ��______��
��2����CuH�м������ϡHC1�����������ɣ��÷�Ӧ�����ӷ���ʽΪ______��
��3������ͭ����Cu2S��FeS�ۺϳɺ�Cu 18%��20%��һ�����ʣ������ۼ������{���´����������ͭ�е�Cu2S������ΪCu2O�����ɵ�Cu2O��Cu2S��Ӧ���ɴ�ͭ������������Ӧ�Ļ�ѧ����ʽ�ֱ���______��______��
��4�������£���0.20 mol ? L-1����ͭ��Һ�м�������������Һ������dz��ɫ������ͭ����������Һ��pH = 6ʱ��c(Cu2+)=______mol.L?1��[��֪��Ksp(CuI)=2.2��10-20(mol/L)3]
��5����0.80 gCuSO4 ? 5H2O��Ʒ���ȷֽ⣬����ˮ�����й����������¶ȵı仯����ͼ��ʾ��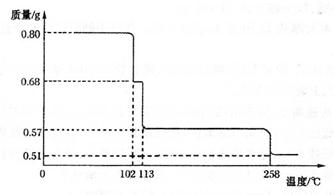
��ȷ��110��Cʱ�������ʵĻ�ѧʽ____________����Ҫ��д���ƶϹ��̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I����������������Ԫ�أ��̷���FeSO4��7H2O��������ȱ����ƶѪҩƷ����Ҫ�ɷݡ�
��1��FeSO4��Һ�ڿ����л����������ʲ������ɫ�������䷢����Ӧ�����ӷ���ʽ�� ��ʵ����������FeSO4��Һʱ������ �Է�ֹ�䱻�������������һ��ʵ��֤��FeSO4��Һ�Ƿ����� ��
��2���ⶨ�̷���Ʒ������ʵ�鲽�裺
a. ��ȡ5.7g��Ʒ���ܽ⣬���250mL��Һ
b.��ȡ25mL������Һ����ƿ��
c.�������ữ��0.01mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ���Ϊ40mL
�������ϲ���ش��������⣺
���������ữ��KMnO4�ζ��յ�ı�־�� ��
�ڼ���������Ʒ��FeSO4��7H2O����������Ϊ ��
II����������泥�NH4��2Fe��SO4��2��6H2O]�������������ױ����������������ڴ�������������
��3����������鱗��ױ�������ԭ�� ��
��4��Ϊ����ֽ����ijɷݣ��������ʵ��װ�ý���ʵ�飬����A��������������ֽ���ȫ��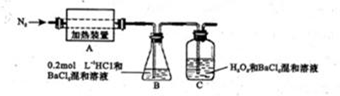
��A�й����ּ��Ƚϳ�ʱ���ͨ�뵪����Ŀ���� ��
��װ��B��BaC12��Һ��������Ϊ�˼���ֽ�������Ƿ���SO3�������ɣ������и����壬�۲쵽�Ĺ���Ϊ ��
��ʵ���У��۲쵽C���а�ɫ�������ɣ���C�з����ķ�ӦΪ �������ӷ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(13�֣�ij�ֵ�ص��������ϣ���ͨ�����з����Ƶã�
�ٳ�ȡһ�����Ĺ�����������Բ������ڹ������ᣬ�����Ⱥ����˫��ˮ������������Һ������24 h,����
�ڽ��������뷴Ӧƿ�У�����������������Һ��80'c���¼���24 ha
����������������ˮ���õ���ɫFeF3����
���ڸ������У���1000CԤ����6 h,��������������Բ��ϻ����ĥ�Ƶ���������
��1������˫��ˮ�������ǣߣߣߣ������ӷ���ʽ��ʾ��
��2������ڵķ�Ӧƿ���ʿ����ǣߣߣߣ�����ţ�
A��������B���մɡ�C�����ķ���ϩ
��3��������ᷴ����Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣ�
��4���Ӱ�ȫ�ĽǶȿ��ǣ���ʵ����������������ʱӦ�ڣߣߣߣ��н���
��5������������Li�ڹ��������������з����û���Ӧ�������ĵ����ɹ�����ʹ�ã��õ�ط�
Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣ�
��6��ȡ������������Ʒ�����������ᣬ��ͨ���״����672 mL C12, Fe2��ǡ�ñ���ȫ������
Fe3ʮ��Ȼ�����Һ���ɣ����������ص�9��6 g���壬���������Ļ�ѧʽΪ�ߣߣߣߡ�
�鿴�𰸺ͽ���>>
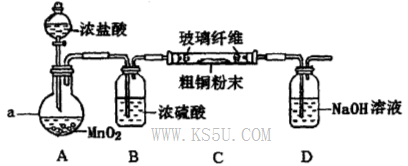
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣��ƵĻ������ڹ�ũҵ���������ǵ��ճ����������Ź㷺��Ӧ�ã�����Na2O2������Ư����DZˮͧ�еĹ�������Na2O2��ǿ�����ԣ�H2���л�ԭ�ԣ�ijͬѧ����������ԭ��Ӧ��֪ʶ�Ʋ�Na2O2��H2�ܷ�Ӧ��Ϊ����֤���Ʋ�������ͬѧ��Ʋ���������ʵ�飬ʵ�鲽����������¡�
����1������ͼ��װ������ͼ�мг�����ʡ�ԣ������װ�õ������ԣ�Ȼ��װ��ҩƷ��
����2����K1��K2������������������װ��Na2O2��Ӳ�ʲ����ܵĹ����У�û�й۲쵽�κ�����
����3��������H2�Ĵ��Ⱥ�ȼ�ƾ��Ƽ��ȣ��۲쵽Ӳ�ʲ�������Na2O2���ۻ�������ɫ�ķ�ĩ��������˰�ɫ���壬�����������ͭδ����ɫ��
����4����Ӧ��ȥ�ƾ��ƣ���Ӳ�ʲ�������ȴ��ر�K1��
����������Ϣ�ش��������⣺
��1����װ��������Ҫ���װ�õ������ԡ��������K2֮ǰװ�������Եķ����������� ������Aװ���Ʊ�������ŵ�����������������д��1�㼴�ɣ���ʵ��������Aװ�û������Ʊ��������������� ��д��1�ּ��ɣ���
��2��ʢװCuSO4ҩƷ������������������ ��Bװ�õ������������������������� ��
��3����������������ȵ�ԭ�������������������������������������� ��
��4������װ��D��Ŀ������������������������ ��
��5��������ʵ����Ƴ�Na2O2��H2��Ӧ�Ļ�ѧ����ʽΪ������������������������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com